Tramapap
Living Well Pharmacy, Inc.
Living Well Pharmacy, Inc.
Tramapap
FULL PRESCRIBING INFORMATION
LWP
381 Van Ness Ave. Suite 1507
Torrance CA, 90501
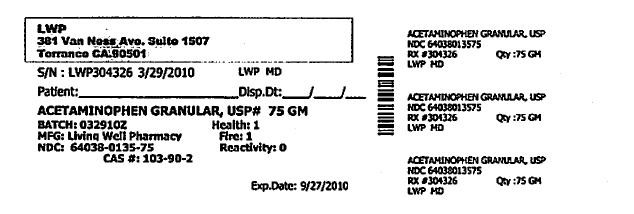
S/N: LWP304326 3/29/2010 LWP,MD
ACETAMINOPHEN GRANULAR, USP #75 GM
BATCH: 03292010Z HEALTH: 1
MFG: LIVING WELL PHARMACY FIRE: 1
NDC: 64038-0135-75 REACTIVITY: 0
LWP
381 Van Ness Ave. Suite 1507
Torrance CA, 90501
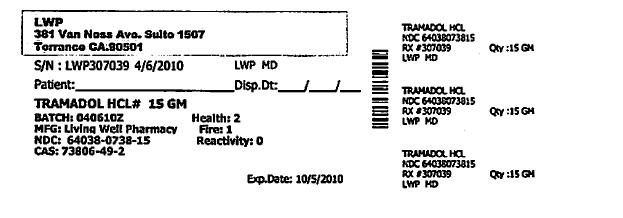
S/N: LWP307039 4/6/2010 LWP,MD
TRAMADOL HCL #15GM
BATCH: 04062010Z HEALTH: 2
MFG: LIVING WELL PHARMACY FIRE: 1
NDC: 64038-0738-15 REACTIVITY: 0
- RX Only
For Prescription Compounding Only
Uses
Equipment
Required supplies needed to compound this kit
| Item |
Quantity |
|---|---|
| Tramadol HCl (Included) |
15 grams |
| Acetaminophen (Included) |
75 grams |
| Lactose Monohydrate Spray Dried Powder ( Included) |
23.25 grams |
| Riboflavin Powder (Included) |
1.5 grams |
| Red/Blue #1 Capsules ( Required Not Included) |
300 Capsules |
|
|
|
Equipment
Recommended supplies not included in this kit
| Item |
Quantity |
|---|---|
| 16 oz Glass Mortar and Pestle |
1 each |
| 300 Capsule Machine Number 2 Notch |
1 |
| Scraper ( Recommended Not Included) |
1 |
| Tamper (Recommended Not Included) |
1 |
| Capsule Locker (Recommended Not Included) |
1 |
|
|
|
Directions
1. Using a 16 oz mortar and pestle triturate powders well to reduce particle size until uniform.
2. Using a 300 capsule machine encapsulate triturate powder mixture into number 1 red/blue capsules
Prior to compounding, store Tramapap Kit at room temperature. Store the final product at room temperature.
Final product for oral use only. Keep out the reach of children. Compounded product, as dispensed,
is stable for at least 180 days or the time remaining on the expiration date of any given ingredient,
which ever is shorter.


TramapapTramadol Hydrochloride, Acetaminophen KIT
| ||||||||||||||||||||||||||||||||||||||||