TRAZODONE
PD-Rx Pharmaceuticals, Inc.
PD-Rx Pharmaceuticals, Inc.
TRAZODONE HYDROCHLORIDE TABLETS, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRAZODONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- TRAZODONE INDICATIONS AND USAGE
- TRAZODONE CONTRAINDICATIONS
- TRAZODONE ADVERSE REACTIONS
- OVERDOSAGE
- TRAZODONE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
FULL PRESCRIBING INFORMATION
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of trazodone HCl or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Trazodone HCl is not approved for use in pediatric patients. (See Warnings: Clinical Worsening and Suicide Risk, Precautions: Information for Patients, and Precautions: Pediatric Use
TRAZODONE DESCRIPTION
Trazodone HCl is an antidepressant chemically unrelated to tricyclic, tetracyclic, or other known antidepressant agents. Trazodone HCl is a triazolopyridine derivative designated as 2-[3-[4-(m-Chlorophenyl)-1-piperazinyl]propyl]s-triazolo[4,3-a]-pyridin-3 (2H)-one monohydrochloride. It is a white to off-white crystalline powder which is sparingly soluble in chloroform and in water. Its molecular weight is 408.3. The molecular formula is C19H22ClN5O•HCl and the structural formula is represented as follows:
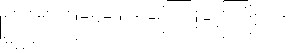
Each tablet, for oral administration, contains 50 mg, 100 mg or 150 mg of trazodone hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, anhydrous lactose, magnesium stearate, microcrystalline cellulose and sodium starch glycolate.
CLINICAL PHARMACOLOGY
The mechanism of trazodone HCl’s antidepressant action in man is not fully understood. In animals, trazodone HCl selectively inhibits its serotonin uptake by brain synaptosomes and potentiates the behavioral changes induced by the serotonin precursor, 5-hydroxytryptophan. Cardiac conduction effects of trazodone HCl in the anesthetized dog are qualitatively dissimilar and quantitatively less pronounced than those seen with tricyclic antidepressants. Trazodone HCl is not a monoamine oxidase inhibitor and, unlike amphetamine type drugs, does not stimulate the central nervous system.
TRAZODONE INDICATIONS AND USAGE
Trazodone hydrochloride tablets are indicated for the treatment of depression. The efficacy of trazodone HCl has been demonstrated in both inpatient and outpatient settings and for depressed patients with and without prominent anxiety. The depressive illness of patients studied corresponds to the Major Depressive Episode criteria of the American Psychiatric Association’s Diagnostic and Statistical Manual, lll.a
Major Depressive Episode implies a prominent and relatively persistent (nearly every day for at least two weeks) depressed or dysphoric mood that usually interferes with daily functioning, and includes at least four of the following eight symptoms: change in appetite, change in sleep, psychomotor agitation or retardation, loss of interest in usual activities or decrease in sexual drive, increased fatigability, feelings of guilt or worthlessness, slowed thinking or impaired concentration, and suicidal ideation or attempts.
TRAZODONE CONTRAINDICATIONS
Trazodone hydrochloride tablets are contraindicated in patients hypersensitive to trazodone HCl.
TRAZODONE ADVERSE REACTIONS
Because the frequency of adverse drug effects is affected by diverse factors (e.g., drug dose, method of detection, physician judgment, disease under treatment, etc.) a single meaningful estimate of adverse event incidence is difficult to obtain. This problem is illustrated by the variation in adverse event incidence observed and reported from the inpatients and outpatients treated with trazodone HCl. It is impossible to determine precisely what accounts for the differences observed.
Clinical Trial Reports
Table 2 below is presented solely to indicate the relative frequency of adverse events reported in representative controlled clinical studies conducted to evaluate the safety and efficacy of trazodone HCl.
The figures cited cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice where patient characteristics and other factors often differ from those which prevailed in clinical trials. These incidence figures, also, cannot be compared with those obtained from other clinical studies involving related drug products and placebo as each group of drug trials is conducted under a different set of conditions.
OVERDOSAGE
Animal Oral LD50: The oral LD50 of the drug is 610 mg/kg in mice, 486 mg/kg in rats, and 560 mg/kg in rabbits.
Signs and Symptoms: Death from overdose has occurred in patients ingesting trazodone HCl and other drugs concurrently (namely, alcohol; alcohol + chloral hydrate + diazepam; amobarbital; chlordiazepoxide; or meprobamate).
The most severe reactions reported to have occurred with overdose of trazodone HCl alone have been priapism, respiratory arrest, seizures, and EKG changes. The reactions reported most frequently have been drowsiness and vomiting. Overdosage may cause an increase in incidence or severity of any of the reported adverse reactions (see ADVERSE REACTIONS).
Treatment
There is no specific antidote for trazodone HCl. Treatment should be symptomatic and supportive in the case of hypotension or excessive sedation. Any patient suspected of having taken an overdose should have the stomach emptied by gastric lavage. Forced diuresis may be useful in facilitating elimination of the drug.
TRAZODONE DOSAGE AND ADMINISTRATION
The dosage should be initiated at a low level and increased gradually, noting the clinical response and any evidence of intolerance. Occurrence of drowsiness may require the administration of a major portion of the daily dose at bedtime or a reduction of dosage. Trazodone HCl should be taken shortly after a meal or light snack. Symptomatic relief may be seen during the first week with optimal antidepressant effects typically evident within two weeks. Twenty-five percent of those who respond to trazodone HCl require more than two weeks (up to four weeks) of drug administration.
Usual Adult Dosage: An initial dose of 150 mg/day in divided doses is suggested. The dose may be increased by 50 mg/day every three to four days. The maximum dose for outpatients usually should not exceed 400 mg/day in divided doses. Inpatients (i.e., more severely depressed patients) may be given up to but not in excess of 600 mg/day in divided doses.
Maintenance: Dosage during prolonged maintenance therapy should be kept at the lowest effective level. Once an adequate response has been achieved, dosage may be gradually reduced, with subsequent adjustment depending on therapeutic response.
Although there has been no systematic evaluation of the efficacy of trazodone beyond 6 weeks, it is generally recommended that a course of antidepressant drug treatment should be continued for several months.
HOW SUPPLIED
Trazodone Hydrochloride Tablets, USP:
50 mg - White, round, scored tablets in bottles of 30, 90, 100, 500, and 1000.
Debossed: PLIVA 433
100 mg - White, round, scored tablets in bottles of 30, 90, 100, 500 and 1000.
Debossed: PLIVA 434
150 mg - White, trapezoidal-shaped tablets, bisected one side to yield two 75 mg units (Debossed PLIVA 441); trisected the other side to yield three 50 mg units (Debossed 50 in each triangular segment); in bottles of 30, 90,100, 500, and 1000.
Dispense in a tight, light-resistant container.
PD-Rx Labels
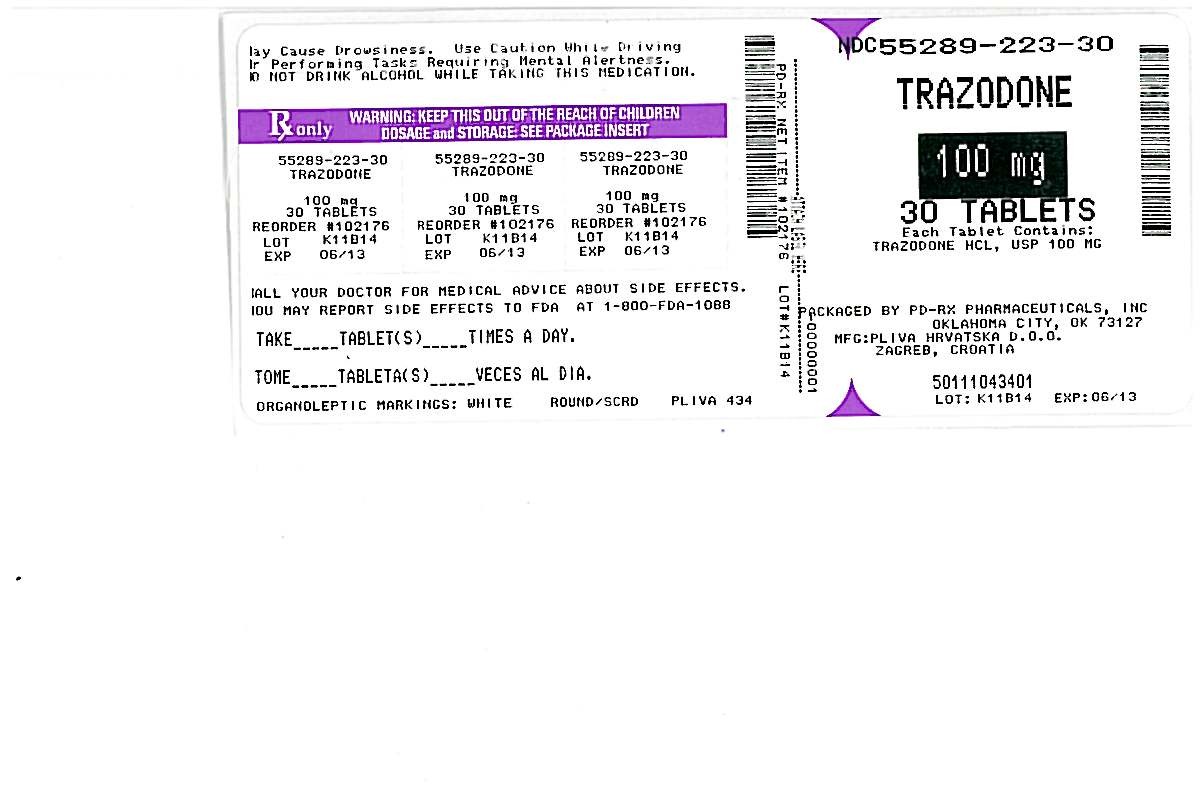
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
REFERENCES
(a) Williams JBW, Ed: Diagnostic and Statistical Manual of Mental Disorders lll, American Psychiatric Association, May, 1980. (b) Lue TF, Physiology of erection and pathophysiology of impotence. In: Wash PC, Retik AB, Stamey TA, Vaughan ED, eds. Campbell’s Urology. Sixth edition. Philadelphia: W.B. Saunders: 1992: 722-725. (c) Goldstein I, Krane RJ, Diagnosis and therapy of erectile dysfunction. In: Wash PC. Retik AB, Stamey TA, Vaughan ED, eds. Campbell’s Urology. Sixth edition. Philadelphia: W.B. Saunders: 1992: 3071-3072. (d) Yealy DM, Hogya PT: Priapism. Emerg Med Clin North Am, 1988: 6:509-520. (e) Banos JE, Bosch F, Farre M. Drug-induced priapism, its aetiology, incidence and treatment. Med Toxicol Adverse Drug Exp. 1989: 4:46-58. (f) O’Brien WM, O’Connor KP, Lynch JH. Priapism: current concepts. Ann Emerg Med. 1989: 980-983. (g) Bardin ED, Krieger JN. Pharmacological priapism: comparison of trazodone- and papaverine-associated cases. Int Urol Nephrol. 1990: 22:147-152.
TRAZODONETRAZODONE HYDROCHLORIDE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||