Tretinoin
Tretinoin gel microsphere, 0.1% and 0.04%
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRETINOIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- TRETINOIN INDICATIONS AND USAGE
- CLINICAL STUDIES
- TRETINOIN CONTRAINDICATIONS
- PRECAUTIONS
- TRETINOIN ADVERSE REACTIONS
- OVERDOSAGE
- TRETINOIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PATIENT INFORMATION
- PRINCIPAL DISPLAY PANEL - 0.1% 20g Tube
- PRINCIPAL DISPLAY PANEL - 0.04% 20g Tube
FULL PRESCRIBING INFORMATION
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE.
TRETINOIN DESCRIPTION
TRETINOIN gel microsphere, 0.1% and 0.04%, is a formulation containing 0.1% or 0.04%, by weight, tretinoin for topical treatment of acne vulgaris. This formulation uses patented methyl methacrylate/glycol dimethacrylate crosspolymer porous microspheres (MICROSPONGE® System) to enable inclusion of the active ingredient, tretinoin, in an aqueous gel. Other components of this formulation are purified water, carbomer 974P (0.04% formulation), carbomer 934P (0.1% formulation), glycerin, disodium EDTA, propylene glycol, sorbic acid, PPG-20 methyl glucose ether distearate, cyclomethicone and dimethicone copolyol, benzyl alcohol, trolamine, and butylated hydroxtoluene.
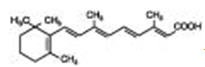
Chemically, tretinoin is all-trans-retinoic acid, also known as (all-E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid. It is a member of the retinoid family of compounds, and a metabolite of naturally occurring Vitamin A. Tretinoin has a molecular weight of 300.44. Tretinoin has the following structure:
CLINICAL PHARMACOLOGY
Tretinoin is a retinoid metabolite of Vitamin A that binds to intracellular receptors in the cytosol and nucleus, but cutaneous levels of tretinoin in excess of physiologic concentrations occur following application of a tretinoin-containing topical drug product.
Although tretinoin activates three members of the retinoid acid (RAR) nuclear receptors (RARα, RARβ, and RARγ) which may act to modify gene expression, subsequent protein synthesis, and epithelial cell growth and differentiation, it has not been established whether the clinical effects of tretinoin are mediated through activation of retinoic acid receptors, other mechanisms, or both.
Mode of Action
Although the exact mode of action of tretinoin is unknown, current evidence suggests that the effectiveness of tretinoin in acne is due primarily to its ability to modify abnormal follicular keratinization. Comedones form in follicles with an excess of keratinized epithelial cells. Tretinoin promotes detachment of cornified cells and the enhanced shedding of corneocytes from the follicle. By increasing the mitotic activity of follicular epithelia, tretinoin also increases the turnover rate of thin, loosely-adherent corneocytes. Through these actions, the comedo contents are extruded and the formation of the microcomedo, the precursor lesion of acne vulgaris, is reduced.
Additionally, tretinoin acts by modulating the proliferation and differentiation of epidermal cells. These effects are mediated by tretinoin's interaction with a family of nuclear retinoic receptors. Activation of these nuclear receptors causes changes in gene expression. The exact mechanisms whereby tretinoin-induced changes in gene expression regulate skin function are not understood.
Pharmacokinetics
Tretinoin is a metabolite of Vitamin A metabolism in man.
Percutaneous absorption, as determined by the cumulative excretion of radiolabeled drug into urine and feces, was assessed in 44 healthy men and women. Estimates of in vivo bioavailability, mean (SD)%, following both single and multiple daily applications, for a period of 28 days with the 0.1% gel, were 0.82 (0.11)% and 1.41 (0.54)%, respectively. The plasma concentrations of tretinoin and its metabolites, 13-cis-retinoic acid, all-trans-4-oxo-retinoic acid, and 13-cis-4-oxo-retinoic acid, generally ranged from 1 to 3 ng/mL and were essentially unaltered after either single or multiple daily applications of TRETINOIN gel microsphere, 0.1%, relative to baseline levels. Clinical pharmacokinetic studies have not been performed with TRETINOIN gel microsphere, 0.04%.
TRETINOIN INDICATIONS AND USAGE
TRETINOIN gel microsphere, 0.1% and 0.04%, is indicated for topical application in the treatment of acne vulgaris. The safety and efficacy of the use of this product in the treatment of other disorders have not been established.
CLINICAL STUDIES
TRETINOIN gel microsphere, 0.1%
In two vehicle-controlled studies, TRETINOIN gel microsphere, 0.1%, applied once daily was significantly more effective than vehicle in reducing the severity of acne lesion counts. The mean reductions in lesion counts from baseline after treatment for 12 weeks are shown in the following table:
| TRETINOIN gel microsphere, 0.1% |
Vehicle Gel | |||
|---|---|---|---|---|
| Study #1 72 pts |
Study #2 71 pts |
Study #1 72 pts |
Study #2 67 pts |
|
| Non-inflammatory lesion counts | 49% | 32% | 22% | 3% |
| Inflammatory lesion counts | 37% | 29% | 18% | 24% |
| Total lesion counts | 45% | 32% | 23% | 16% |
TRETINOIN gel microsphere, 0.1% was also significantly superior to the vehicle in the investigator's global evaluation of the clinical response. In Study #1, thirty-five percent (35%) of patients using TRETINOIN gel microsphere, 0.1%, achieved an excellent result, as compared to eleven percent (11%) of patients on the vehicle control. In Study #2, twenty-eight percent (28%) of patients using TRETINOIN gel microsphere, 0.1%, achieved an excellent result, as compared to nine percent (9%) of the patients on the vehicle control.
TRETINOIN gel microsphere, 0.04%
In two vehicle-controlled clinical studies, TRETINOIN gel microsphere, 0.04%, applied once daily, was more effective (p< 0.05) than vehicle in reducing the acne lesion counts. The mean reductions in lesion counts from baseline after treatment for 12 weeks are shown in the following table:
| TRETINOIN gel microsphere, 0.04% |
Vehicle Gel | |||
|---|---|---|---|---|
| Study #1 108 pts |
Study #2 111 pts |
Study #1 110 pts |
Study #2 103 pts |
|
| Non-inflammatory lesion counts | 37% | 29% | -2% |
14% |
| Inflammatory lesion counts | 44% | 41% | 13% | 30% |
| Total lesion counts | 40% | 35% | 8% | 20% |
TRETINOIN gel microsphere, 0.04%, was also superior (p<0.05) to the vehicle in the investigator's global evaluation of the clinical response. In Study #1, fourteen percent (14%) of patients using TRETINOIN gel microsphere, 0.04%, achieved an excellent result compared to five percent (5%) of patients on vehicle control. In Study #2, nineteen percent (19%) of patients using TRETINOIN gel microsphere, 0.04%, achieved an excellent result compared to nine percent (9%) of patients on vehicle control.
No studies were conducted comparing the efficacy of TRETINOIN gel microsphere, 0.04% to TRETINOIN gel microsphere, 0.1%. There is no evidence that TRETINOIN gel microsphere, 0.1% is more efficacious than TRETINOIN gel microsphere, 0.04% or that TRETINOIN gel microsphere, 0.04% is safer than TRETINOIN gel microsphere, 0.1%.
TRETINOIN CONTRAINDICATIONS
This drug is contraindicated in individuals with a history of sensitivity reactions to any of its components. It should be discontinued if hypersensitivity to any of its ingredients is noted.
PRECAUTIONS
General
- The skin of certain individuals may become excessively dry, red, swollen, or blistered. If the degree of irritation warrants, patients should be directed to temporarily reduce the amount or frequency of application of the medication, discontinue use temporarily, or discontinue use all together. Efficacy at reduced frequencies of application had not been established. If a reaction suggesting sensitivity occurs, use of the medication should be discontinued. Excessive skin dryness may also be experienced; if so, use of an appropriate emollient during the day may be helpful.
- Unprotected exposure to sunlight, including sunlamps, should be minimized during the use of TRETINOIN gel microsphere, 0.1% and 0.04%, and patients with sunburn should be advised not to use the product until fully recovered because of heightened susceptibility to sunlight as a result of the use of tretinoin. Patients who may be required to have considerable sun exposure due to occupation and those with inherent sensitivity to the sun should exercise particular caution. Use of sunscreen products (SPF 15) and protective clothing over treated areas are recommended when exposure cannot be avoided.
- Weather extremes, such as wind or cold, also may be irritating to patients under treatment with tretinoin.
- TRETINOIN gel microsphere, 0.1% and 0.04%, should be kept away from the eyes, the mouth, paranasal creases of the nose, and mucous membranes.
- Tretinoin has been reported to cause severe irritation on eczematous skin and should be used with utmost caution in patients with this condition.
Information for Patients
See Patient Information Leaflet.
Drug Interactions
Concomitant topical medication, medicated or abrasive soaps and cleansers, products that have a strong drying effect, products with high concentrations of alcohol, astringents, or spices should be used with caution because of possible interaction with tretinoin. Avoid contact with the peel of limes. Particular caution should be exercised with the concomitant use of topical over-the-counter acne preparations containing benzoyl peroxide, sulfur, resorcinol, or salicylic acid with TRETINOIN gel microsphere, 0.1% and 0.04%. It also is advisable to allow the effects of such preparations to subside before use of TRETINOIN gel microsphere, 0.1% and 0.04%, is begun.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 91-week dermal study in which CD-1 mice were administered 0.017% and 0.035% formulations of tretinoin, cutaneous squamous cell carcinomas and papillomas in the treatment area were observed in some female mice. These concentrations are near the tretinoin concentration of these clinical formulations (0.04% and 0.1%). A dose-related incidence of liver tumors in male mice was observed at those same doses. The maximum systemic doses associated with the administered 0.017% and 0.035% formulations are 0.5 and 1.0 mg/kg/day, respectively. These doses are two and four times the maximum human systemic dose applied topically, when normalized for total body surface area. The biological significance of these findings is not clear because they occurred at doses that exceeded the dermal maximally tolerated dose (MTD) of tretinoin and because they were within the background natural occurrence rate for these tumors in this strain of mice. There was no evidence of carcinogenic potential when 0.025 mg/kg/day of tretinoin was administered topically to mice (0.1 times the maximum human systemic dose, normalized for total body surface area). For purposes of comparisons of the animal exposure to systemic human exposure, the maximum human systemic dose applied topically is defined as 1 gram of TRETINOIN gel microsphere, 0.1%, applied daily to a 50 kg person (0.02 mg tretinoin/kg body weight).
Dermal carcinogenicity testing has not been performed with TRETINOIN gel microsphere, 0.04% or 0.1%.
Studies in hairless albino mice suggest that concurrent exposure to tretinoin may enhance the tumorigenic potential of carcinogenic doses of UVB and UVA light from a solar simulator. This effect has been confirmed in a later study in pigmented mice, and dark pigmentation did not overcome the enhancement of photocarcinogenesis by 0.05% tretinoin. Although the significance of these studies to humans is not clear, patients should minimize exposure to sunlight or artificial ultraviolet irradiation sources.
The mutagenic potential of tretinoin was evaluated in the Ames assay and in the in vivo mouse micronucleus assay, both of which were negative.
The components of the microspheres have shown potential for genetic toxicity and teratogenesis. EGDMA, a component of the excipient acrylates copolymer, was positive for induction of structural chromosomal aberrations in the in vitro chromosomal aberration assay in mammalian cells in the absence of metabolic activation, and negative for genetic toxicity in the Ames assay, the HGPRT forward mutation assay, and the mouse micronucleus assay.
In dermal Segment I fertility studies of another tretinoin formulation in rats, slight (not statistically significant) decreases in sperm count and motility were seen at 0.5 mg/kg/day (4 times the maximum human systemic dose applied topically, and normalized for total body surface area), and slight (not statistically significant) increases in the number and percent of nonviable embryos in females treated with 0.25 mg/kg/day (2 times the maximum human systemic dose applied topically and normalized for total body surface area) and above were observed. In oral Segment I and Segment III studies in rats with tretinoin, decreased survival of neonates and growth retardation were observed at doses in excess of 2 mg/kg/day (17 times the human topical dose normalized for total body surface area.)
Dermal fertility and perinatal development studies with TRETINOIN gel microsphere, 0.1% or 0.04%, have not been performed in any species.
Pregnancy
Teratogenic Effects
Pregnancy Category C
In a study of pregnant rats treated with topical application of TRETINOIN gel microsphere, 0.1%, at doses of 0.5 to 1 mg/kg/day on gestation days 6-15 (4 to 8 times the maximum human systemic dose of tretinoin normalized for total body surface area after topical administration of TRETINOIN gel microsphere, 0.1%) some alterations were seen in vertebrae and ribs of offspring. In another study, pregnant New Zealand white rabbits were treated with TRETINOIN gel microsphere, 0.1%, at doses of 0.2, 0.5, and 1.0 mg/kg/day, administered topically for 24 hours a day while wearing Elizabethan collars to prevent ingestion of the drug. There appeared to be increased incidences of certain alterations, including domed head and hydrocephaly, typical of retinoid-induced fetal malformations in this species, at 0.5 and 1.0 mg/kg/day. Similar malformations were not observed at 0.2 mg/kg/day, 3 times the maximum human systemic dose of tretinoin after topical administration of TRETINOIN gel microsphere, 0.1%, normalized for total body surface area. In a repeat study of the highest topical dose (1.0 mg/kg/day) in pregnant rabbits, these effects were not seen, but a few alterations that may be associated with tretinoin exposure were seen. Other pregnant rabbits exposed topically for six hours to 0.5 or 0.1 mg/kg/day tretinoin while restrained in stocks to prevent ingestion, did not show any teratogenic effects at doses up to 17 times (1.0 mg/kg/day) the maximum human systemic dose after topical administration of TRETINOIN gel microsphere, 0.1%, adjusted for total body surface area, but fetal resorptions were increased at 0.5 mg/kg. In addition, topical tretinoin in non TRETINOIN gel microsphere formulations was not teratogenic in rats and rabbits when given in doses of 42 and 27 times the maximum human systemic dose after topical administration of TRETINOIN gel microsphere, 0.1%, normalized for total body surface area, respectively, (assuming a 50 kg adult applied a daily dose of 1.0 g of 0.1% gel topically). At these topical doses, however, delayed ossification of several bones occurred in rabbits. In rats, a dose-dependent increase of supernumerary ribs was observed.
Oral tretinoin has been shown to be teratogenic in rats, mice, rabbits, hamsters, and subhuman primates. Tretinoin was teratogenic in Wistar rats when given orally or topically in doses greater than 1 mg/kg/day (8 times the maximum human systemic dose normalized for total body surface area). However, variations in teratogenic doses among various strains of rats have been reported. In the cynomolgus monkey, which metabolically is more similar to humans than other species in its handling of tretinoin, fetal malformations were reported for doses of 10 mg/kg/day or greater, but none were observed at 5 mg/kg/day (83 times the maximum human systemic dose normalized for total body surface area), although increased skeletal variations were observed at all doses. Dose-related increases in embryolethality and abortion also were reported. Similar results have also been reported in pigtail macaques.
Topical tretinoin in animal teratogenicity tests has generated equivocal results. There is evidence for teratogenicity (shortened or kinked tail) of topical tretinoin in Wistar rats at doses greater than 1 mg/kg/day (8 times the maximum human systemic dose normalized for total body surface area). Anomalies (humerus: short 13%, bent 6%, os parietal incompletely ossified 14%) have also been reported when 10 mg/kg/day was topically applied. Supernumerary ribs have been a consistent finding in rats when dams were treated topically or orally with retinoids.
There are no adequate and well-controlled studies in pregnant women. TRETINOIN gel microsphere, 0.1% and 0.04% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
With widespread use of any drug, a small number of birth defect reports associated temporally with the administration of the drug would be expected by chance alone. Thirty human cases of temporally associated congenital malformations have been reported during two decades of clinical use of TRETINOIN gel microsphere, 0.1% and 0.04%. Although no definite pattern of teratogenicity and no causal association has been established from these cases, five of the reports describe the rare birth defect category holoprosencephaly (defects associated with incomplete midline development of the forebrain). The significance of these spontaneous reports in terms of risk to the fetus is not known.
Non-Teratogenic Effects
Topical tretinoin has been shown to be fetotoxic in rabbits when administered 0.5 mg/kg/day (8 times the maximum human systemic dose applied topically and normalized for total body surface area), resulting in fetal resorptions and variations in ossification. Oral tretinoin has been shown to be fetotoxic, resulting in skeletal variations and increased intrauterine death in rats when administered 2.5 mg/kg/day (21 times the maximum human systemic dose applied topically and normalized for total body surface area).
There are, however, no adequate and well-controlled studies in pregnant women.
Animal Toxicity Studies
In male mice treated topically with TRETINOIN gel microsphere, 0.1%, at 0.5, 2.0, or 5.0 mg/kg/day tretinoin (2, 8, or 21 times the maximum human systemic dose after topical administration of TRETINOIN gel microsphere, 0.1%, normalized for total body surface area) for 90 days, a reduction in testicular weight, but with no pathological changes were observed at the two highest doses. Similarly, in female mice there was a reduction in ovarian weights, but without any underlying pathological changes, at 5.0 mg/kg/day (21 times the maximum human dose). In this study there was a dose-related increase in the plasma concentration of tretinoin 4 hours after the first dose. A separate toxicokinetic study in mice indicates that systemic exposure is greater after topical application to unrestrained animals than to restrained animals, suggesting that the systemic toxicity observed is probably related to ingestion. Male and female dogs treated with TRETINOIN gel microsphere, 0.1%, at 0.2, 0.5, or 1.0 mg/kg/day tretinoin (5, 12, or 25 times the maximum human systemic dose after topical administration of TRETINOIN gel microsphere, 0.1%, normalized for total body surface area, respectively) for 90 days showed no evidence of reduced testicular or ovarian weights or pathological changes.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TRETINOIN gel microsphere, 0.1% or 0.04%, is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children below the age of 12 have not been established.
Geriatric Use
Safety and effectiveness in a geriatric population have not been established. Clinical studies of TRETINOIN gel microspere, 0.1% and 0.04% did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently from younger subjects
TRETINOIN ADVERSE REACTIONS
Irritation Potential
Acne clinical trial results
In separate clinical trials for each concentration, acne patients treated with TRETINOIN gel microsphere, 0.1% or 0.04%, analysis over the twelve week period showed that cutaneous irritation scores for erythema, peeling, dryness, burning/stinging, or itching peaked during the initial two weeks of therapy, decreasing thereafter.
Approximately half of the patients treated with TRETINOIN gel microsphere, 0.04% had cutaneous irritation at Week 2. Of those patients who did experience cutaneous side effects, most had signs or symptoms that were mild in severity (severity was ranked on a 4-point ordinal scale: 0=none, 1=mild, 2=moderate, and 3=severe). Less than 10% of patients experienced moderate cutaneous irritation and there was no severe irritation at Week 2.
In studies on TRETINOIN gel microsphere, 0.04%, throughout the treatment period the majority of patients experienced some degree of irritation (mild, moderate, or severe) with 1% (2/225) of patients having scores indicative of a severe irritation rating; and 1.3% (3/225) of patients treated with TRETINOIN gel microsphere, 0.04%, discontinued treatment due to irritation, which included dryness in one patient and peeling and urticaria in another.
In studies on TRETINOIN gel microsphere, 0.1%, no more than 3% of patients had cutaneous irritation scores indicative of severe irritation rating; although, 6% (14/224) of patients treated with TRETINOIN gel microsphere, 0.1% discontinued treatment due to irritation. Of these 14 patients, four have severe irritation after 3 to 5 days of treatment, with blistering in one patient.
Results in studies of subjects without acne
In a half-face comparison trial conducted for up to 14 days in women with sensitive skin, but without acne, TRETINOIN gel microsphere, 0.1% was statistically less irritating than tretinoin cream, 0.1%. In addition, a cumulative 21 day irritation evaluation in subjects with normal skin showed that TRETINOIN gel microsphere, 0.1%, had a lower irritation profile than tretinoin cream, 0.1%. The clinical significance of these irritation studies for patients with acne is not established. Comparable effectiveness of TRETINOIN gel microsphere, 0.1% and tretinoin cream, 0.1%, has not been established. The lower irritancy of TRETINOIN gel microsphere, 0.1% in subjects without acne may be attributable to the properties of its vehicle. The contribution of decreased irritancy by the MICROSPONGE System has not been established. No irritation studies have been performed to compare TRETINOIN gel microsphere, 0.04%, with either TRETINOIN gelmicrosphere, 0.1%, or tretinoin cream, 0.1%.
The skin of certain sensitive individuals may become excessively red, edematous, blistered, or crusted. If these effects occur, the medication should either be discontinued until the integrity of the skin is restored, or the medication should be adjusted to a level the patient can tolerate. However, efficacy has not been established for lower dosing frequencies (see DOSAGE AND ADMINISTRATION Section).
True contact allergy to topical tretinoin is rarely encountered. Temporary hyper-or hypopigmentation has been reported with repeated application of tretinoin. Some individuals have been reported to have heightened susceptibility to sunlight while under treatment with tretinoin.
OVERDOSAGE
TRETINOIN gel microsphere, 0.1% and 0.04%, is intended for topical use only. If medication is applied excessively, no more rapid or better results will be obtained and marked redness, peeling, or discomfort may occur. Oral ingestion of large amounts of the drug may lead to the same side effects as those associated with excessive oral intake of Vitamin A.
TRETINOIN DOSAGE AND ADMINISTRATION
TRETINOIN gel microsphere, 0.1% and 0.04%, should be applied once a day, in the evening, to the skin where acne lesions appear, using enough to cover the entire affected area lightly. Application of excessive amounts of gel may result in "caking" of the gel, and will not provide incremental efficacy.
A transitory feeling of warmth or slight stinging may be noted on application. In cases where it has been necessary to temporarily discontinue therapy or to reduce the frequency of application, therapy may be resumed or the frequency of application increased as the patient becomes able to tolerate the treatment. Frequency of application should be closely monitored by careful observation of the clinical therapeutic response and skin tolerance. Efficacy has not been established for less than once daily dosing frequencies.
During the early weeks of therapy, an apparent exacerbation of inflammatory lesions may occur. If tolerated, this should not be considered a reason to discontinue therapy. Therapeutic results may be noticed after two weeks, but more than seven weeks of therapy are required before consistent beneficial effects are observed.
Patients treated with TRETINOIN gel microsphere, 0.1% and 0.04%, may use cosmetics, but the areas to be treated should be cleansed thoroughly before the medication is applied.
HOW SUPPLIED
TRETINOIN gel microsphere, 0.1% tube:
20g (NDC 68682-513-82),
45g (NDC 68682-513-84).
TRETINOIN gel microsphere, 0.1% pump:
50g (NDC 68682-513-85)
TRETINOIN gel microsphere, 0.04% tube:
20g (NDC 68682-514-92),
45g (NDC 68682-514-94).
TRETINOIN gel microsphere, 0.04% pump:
50g (NDC 68682-514-95)
Storage Conditions
Store at 15°-25°C (59°-77°F).
Rx only.
Distributed by: Oceanside Pharmaceuticals,
a division of Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807
Manufactured by: Ortho Pharmaceuticals a division of Janssen Ortho LLC,
Manati, Puerto Rico 00674
MICROSPONGE® is a registered trademark of AMCOL International Corporation.
March, 2012
12318200
12320000
PATIENT INFORMATION
TRETINOIN gel microsphere, 0.1% and 0.04%
For Topical Use Only
Read this information carefully before you start to use your medicine and each time you get more medicine. There may be new information about this medicine when you get your prescription renewed. This leaflet provides a summary of the information about TRETINOIN gel microsphere, 0.1% and 0.04%. It does not include everything there is to know about your medicine. This information does not take the place of talking with your doctor; so, if you have any questions or are not sure about something, ask your doctor or pharmacist.
What is the most important information I should know about TRETINOIN gel microsphere, 0.1% and 0.04%?
Tell your doctor if you are pregnant, think you are pregnant, trying to get pregnant, or are breastfeeding. If you become pregnant while using TRETINOIN gel microsphere, 0.1% and 0.04%, contact your doctor right away.
TRETINOIN gel microsphere, 0.1% and 0.04% may make your skin very dry, red, swollen or blistered. Contact your doctor if these side effects bother you.
Use other acne medicines with TRETINOIN gel microsphere, 0.1% and 0.04% only on your doctor's advice and follow your doctor's instructions carefully. The medicines you have used in the past might cause too much redness or peeling.
Avoid sunlight or sunlamps and medicines that may make you more sensitive to sunlight. Your doctor can help you find out if you are taking medicines that may make you more sensitive to sunlight. (See "Who should not use TRETINOIN gel microsphere, 0.1% and 0.04%? " and "What should I avoid while using TRETINOIN gel microsphere, 0.1% and 0.04%?")
What is TRETINOIN gel microsphere, 0.1% and 0.04%?
TRETINOIN gel microsphere, 0.1% and 0.04% is a prescription medicine that you put on your skin to treat acne. Acne is a condition in which the skin has blackheads, whiteheads, and other pimples.
Who should not use TRETINOIN gel microsphere, 0.1% and 0.04%?
Do not use TRETINOIN gel microsphere, 0.1% and 0.04% if:
- you are sunburned. Do not use TRETINOIN gel microsphere, 0.1% and 0.04% until your skin has fully recovered.
- you have eczema or other skin conditions.TRETINOIN gel microsphere, 0.1% and 0.04% can cause severe irritation if used on eczema.
- you are allergic to any of the ingredients in TRETINOIN gel microsphere, 0.1% and 0.04%.
The active ingredient is tretinoin. See the list of other ingredients at the end of this leaflet.
Tell your doctor before using TRETINOIN gel microsphere, 0.1% and 0.04% if:
- you are pregnant, planning to become pregnant, or may be pregnant.
- you are breastfeeding. We do not know if TRETINOIN gel microsphere, 0.1% and 0.04% can pass through your milk to the baby.
- you are using any other medicines to treat your acne. Do not use other medicines unless they are recommended by your doctor. Other acne medicines used with TRETINOIN gel microsphere, 0.1% and 0.04% may make your face more likely to be dry and red and cause it to peel.
- you are taking medicines for other health conditions. Some medicines may make your skin more sensitive to sunlight. Tell your doctor about all medicines that you are taking. (See "Who should not use TRETINOIN gel microsphere, 0.1% and 0.04%?" and " What should I avoid while using TRETINOIN gel microsphere, 0.1% and 0.04%?")
How should I use TRETINOIN gel microsphere, 0.1% and 0.04%?
Wash your skin with mild, non-medicated soap and dry your skin gently. Apply TRETINOIN gel microsphere, 0.1% and 0.04% once a day in the evening, or as directed by your doctor.
Tube: Squeeze a small amount of TRETINOIN gel microsphere, 0.1% and 0.04% (about the size of a pea) on your fingertip. Dab TRETINOIN gel microsphere, 0.1% and 0.04% on your forehead, chin, and both cheeks. Spread TRETINOIN gel microsphere, 0.1% and 0.04% evenly over the entire surface of your face by gently smoothing it into your skin.
Pump: Fully depress the pump twice to dispense a small amount of TRETINOIN gel microsphere, 0.1% and 0.04% (about the size of a pea) onto your fingertip. Dab TRETINOIN gel microsphere, 0.1% and 0.04% on your forehead, chin and both cheeks. Spread TRETINOIN gel microsphere, 0.1% and 0.04% evenly over the entire surface of your face by gently smoothing it into your skin.

Do not put TRETINOIN gel microsphere, 0.1% and 0.04% near your mouth, eyes, or open sores, or on the corners of your nose. Spread TRETINOIN gel microsphere, 0.1% and 0.04% away from these areas when putting TRETINOIN gel microsphere, 0.1% and 0.04% on your skin.
Do not use more TRETINOIN gel microsphere, 0.1% and 0.04% than your doctor has prescribed. Do not use TRETINOIN gel microsphere, 0.1% and 0.04% more often than your doctor has told you. Too much TRETINOIN gel microsphere, 0.1% and 0.04% may irritate or increase the irritation of your skin, and will not give faster or better results.
You can use a facial cream or lotion each morning after washing your face. It should contain a sun protection factor (SPF) of 15 or higher. Follow your doctor's advice because you need to use a cream or lotion that will not make your acne worse. You may use cosmetics with TRETINOIN gel microsphere, 0.1% and 0.04% However, clean your face before using cosmetics and remove cosmetics from your skin before using TRETINOIN gel microsphere, 0.1% and 0.04%. Talk to your doctor about recommended cosmetics.
You may not see improvement right away. TRETINOIN gel microsphere, 0.1% and 0.04% may work better for some patients than for others. Keep using TRETINOIN gel microsphere, 0.1% and 0.04% even after your acne improves. You may notice some results after two weeks, but more than seven weeks of using TRETINOIN gel microsphere, 0.1% and 0.04% are needed before you see the full benefit.
Early in therapy, you may notice new pimples. At this stage, it is important to continue using TRETINOIN gel microsphere, 0.1% and 0.04%.
Once your acne is under control you should continue regular application of TRETINOIN gel microsphere, 0.1% and 0.04% until your doctor instructs otherwise.
What should I avoid while using TRETINOIN gel microsphere, 0.1% and 0.04%?
Spend as little time as possible in the sun. Use a daily sunscreen with a SPF 15 rating or higher, sun protective clothing, and a wide brimmed hat to protect you from sunlight. When outside, even on hazy days, areas treated with TRETINOIN gel microsphere, 0.1% and 0.04% should be protected. Do not use sunlamps. TRETINOIN gel microsphere, 0.1% and 0.04% may make you get sunburned more easily. If you do get sunburned, stop using TRETINOIN gel microsphere, 0.1% and 0.04% until your skin is completely back to normal. Talk to your doctor about how to protect your skin if you must be in sunlight a lot.
Avoid cold weather and wind as much as possible and use clothing to protect you from the weather. Skin treated with TRETINOIN gel microsphere, 0.1% and 0.04% may dry out or get windburned more easily.
Avoid skin products that may dry or irritate your skin. Such products are those that contain astringents, alcohol, or spices and include certain medicated soaps, shampoos and hair permanent solutions. Avoid contact with the peel of limes. Your skin may become very dry, red, swollen, blistered, or crusted with these products. If you get severe skin irritation or skin irritation that will not go away, stop using TRETINOIN gel microsphere, 0.1% and 0.04% and call your doctor. You should talk to your doctor about the use of all skin care products while using TRETINOIN gel microsphere, 0.1% and 0.04%.
Avoid washing your face too often and do not scrub your face hard when washing it. Use a mild, non-medicated soap and wash gently and pat dry.
Talk to your doctor about using other acne medicines while using TRETINOIN gel microsphere, 0.1% and 0.04% as they could cause redness or peeling.
What are the possible side effects of TRETINOIN gel microsphere, 0.1% and 0.04%?
A brief feeling of warmth or stinging may be normal when you apply TRETINOIN gel microsphere, 0.1% and 0.04%.
The most common side effect with TRETINOIN gel microsphere, 0.1% and 0.04% is skin irritation. This can include skin redness, burning, stinging, itching, dryness, and peeling. Some of these side effects may go away or bother you less after you have used TRETINOIN gel microsphere, 0.1% and 0.04% for a few weeks. Tell your doctor if these side effects become a problem. Your doctor may change your dose of TRETINOIN gel microsphere, 0.1% and 0.04%. However, the effectiveness of TRETINOIN gel microsphere, 0.1% and 0.04% when used less than once a day has not been proven.
There may be some mild discomfort or peeling during the early weeks of treatment. Some patients also notice that their skin begins to take on a blush. These reactions happen to about half of the people using TRETINOIN gel microsphere, 0.1% and 0.04%. If this happens to you, it is just your skin getting used to TRETINOIN gel microsphere, 0.1% and 0.04%. This usually improves by 4 weeks after starting treatment. These reactions can usually be lessened by following instructions carefully. If the discomfort is a problem, talk to your doctor.
Call your doctor right away if your face becomes very dry, red, swollen, or blistered. Your doctor may ask you to stop using TRETINOIN gel microsphere, 0.1% and 0.04% for a while, change the amount of TRETINOIN gel microsphere, 0.1% and 0.04% you are using, or change the times that you use TRETINOIN gel microsphere, 0.1% and 0.04%.
These are not all the side effects possible with TRETINOIN gel microsphere, 0.1% and 0.04%. For more information, ask your doctor or pharmacist.
General Information about TRETINOIN gel microsphere, 0.1% and 0.04%.
This medicine is for your use only. Do not allow anyone else to use this medicine. Medicines are sometimes prescribed for conditions not mentioned in patient information leaflets. Do not use TRETINOIN gel microsphere, 0.1% and 0.04% for a condition for which it was not prescribed by your doctor.
What are the ingredients of TRETINOIN gel microsphere, 0.1% and 0.04%?
The active ingredient is tretinoin. The inactive ingredients are purified water, carbomer 974P (0.04% formulation), carbomer 934P (0.1% formulation), glycerin, disodium EDTA, propylene glycol, sorbic acid, PPG-20 methyl glucose ether distearate, cyclomethicone and dimethicone copolyol, benzyl alcohol, trolamine, and butylated hydroxytoluene.
How do I store TRETINOIN gel microsphere, 0.1% and 0.04%?
TRETINOIN gel microsphere, 0.1% and 0.04% should be stored at room temperature, 15°-25°C (59°-77°F).
Where can I get more information about TRETINOIN gel microsphere, 0.1% and 0.04%?
You can ask your doctor or pharmacist for the information about TRETINOIN gel microsphere, 0.1% and 0.04% that is written for health professionals. You can also contact Ortho Dermatologics by calling their toll-free number: 877-738-4624. Call between 9:00 a.m. and 3:00 p.m. Eastern Time, Monday through Friday. Or you can visit our website: www.retinamicro.com.
Distributed by: Oceanside Pharmaceuticals,
a division of Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807
Manufactured by: Ortho Pharmaceuticals a division of Janssen Ortho LLC,
Manati, Puerto Rico 00674
MICROSPONGE® is a registered trademark of AMCOL International Corporation.
March, 2012
12318200
PRINCIPAL DISPLAY PANEL - 0.1% 20g Tube
NDC 68682-513-82
TRETINOIN gel
microsphere, 0.1%
For Topical Use Only
Dosage: See package insert.
OCEANSIDE® PHARMACEUTICALS

PRINCIPAL DISPLAY PANEL - 0.04% 20g Tube
NDC 68682-514-92
TRETINOIN gel
microsphere, 0.04%
For Topical Use Only
Dosage: See package insert.
OCEANSIDE® PHARMACEUTICALS

TretinoinTretinoin GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TretinoinTretinoin GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||