Triadine
H and P Industries, Inc. dba Triad Group
H and P Industries, Inc. dba Triad Group
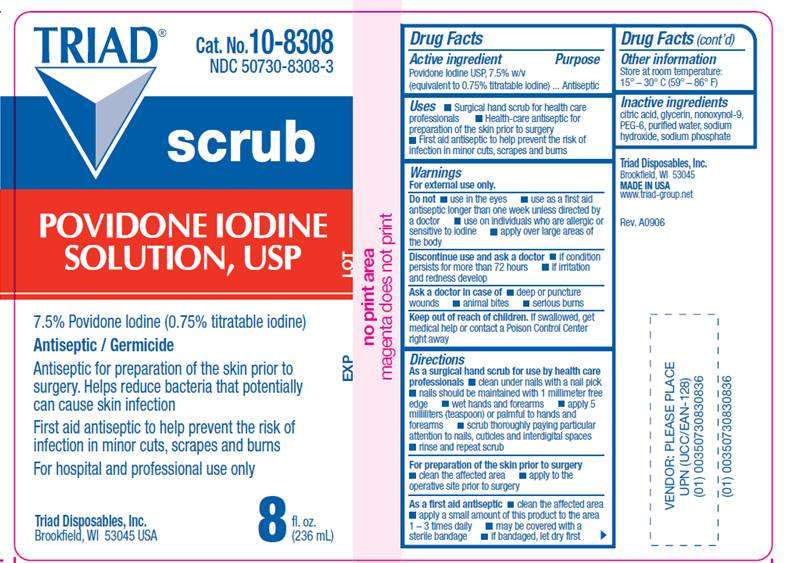
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- LABEL INFORMATION
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
Povidone Iodine USP 7.5% w/v (equivalent to 0.75% titratable iodine)
PURPOSE
Antiseptic
USES
- Surgical hand scrub for health care processionals
- Health Care Antiseptic for preparation of the skin prior to surgery
- First Aid Antiseptic to help prevent the risk of infection in minor cuts, scrapes and burns
WARNINGS
For external use only.
Do not
- use in the eyes
- apply over large areas of the body
- use on individuals who are allergic or sensitive to iodine
- use as a first aid antiseptic for longer than 1 week unless directed by a doctor
Ask a doctor in case of
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor
- if condition persists for more than 72 hours
- if irritation and redness develop
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
As a surgical hand scrub for use by health care professionals- clean under nails with a nail pick
- nails should be maintained with 1 millimeter free edge
- wet hands and forearms
- apply milliliter (teaspoon) or palmful to hands and forearms
- scrub thoroughly paying particular attention to nails, cuticles and interdigital spaces
- rinse and repeat scrub
- clean the affected area
- apply to the operative site prior to surgery
- clean the affected area
- apply a small amount of this product to the area 1-3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
OTHER INFORMATION
INACTIVE INGREDIENTS
LABEL INFORMATION
Antiseptic / Germicide
Triadinepovidone-iodine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!