Triamterene and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRIAMTERENE AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TRIAMTERENE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- TRIAMTERENE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TRIAMTERENE AND HYDROCHLOROTHIAZIDE DESCRIPTION
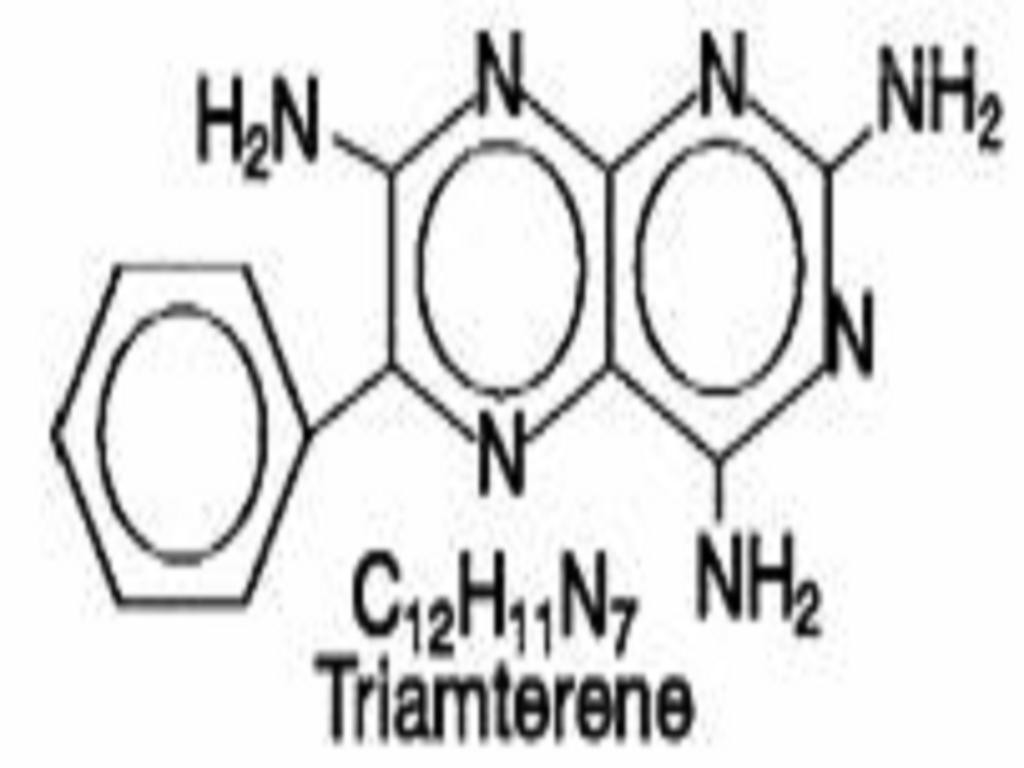

CLINICAL PHARMACOLOGY
Hydrochlorothiazide:
Triamterene:
WARNINGS
INDICATIONS & USAGE
This fixed combination drug is not indicated for the initial therapy of edema or hypertension except in individuals in whom the development of hypokalemia cannot be risked.Usage in Pregnancy:
TRIAMTERENE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
HyperkalemiaAntikaliuretic Therapy or Potassium Supplementation:
Impaired Renal Function:
Hypersensitivity:
WARNINGS
HyperkalemiaAbnormal elevation of serum potassium levels (greater than or equal to 5.5 mEq/liter) can occur with all potassium-conserving diuretic combinations, including triamterene and hydrochlorothiazide. Hyperkalemia is more likely to occur in patients with renal impairment, diabetes (even without evidence of renal impairment) or elderly or severely ill patients. Since uncorrected hyperkalemia may be fatal, serum potassium levels must be monitored at frequent intervals especially in patients first receiving triamterene and hydrochlorothiazide, when dosages are changed, or with any illness that may influence renal function.
CONTRAINDICATIONS
PRECAUTIONS, Drug Interactions
Metabolic or Respiratory Acidosis:
PRECAUTIONS
GeneralElectrolyte imbalance and BUN increases:
Hepatic Coma:
Renal Stones:
Folic Acid Deficiency:
Hyperuricemia:
Metabolic and Endocrine Effects:
Hypersensitivity:
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis:Triamterene:
Hydrochlorothiazide:
Mutagenesis:
Triamterene:
Hydrochlorothiazide:
Impairment of Fertility:
PREGNANCY
Teratogenic EffectsTriamterene:
Hydrochlorothiazide:
Nonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
TRIAMTERENE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Gastrointestinal:
Central Nervous System:
Cardiovascular:
Renal:
Hematologic:
Ophthalmic:
Hypersensitivity:
Other:
Altered Laboratory Findings:
Serum ElectrolytesWARNINGSPRECAUTIONS
Creatinine, Blood Urea Nitrogen:
Glucose:PRECAUTIONS
Serum Uric Acids, PBI and Calcium:PRECAUTIONS
Other:
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGS
PRECAUTIONS, Drug Interactions
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Triamterene and HydrochlorothiazideTriamterene and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!