Tucks
Johnson & Johnson Healthcare Products, Division of McNEIL-PPC, Inc.
Tucks Fast Relief Spray
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Tucks Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 14.8 mL Bottle Label
FULL PRESCRIBING INFORMATION
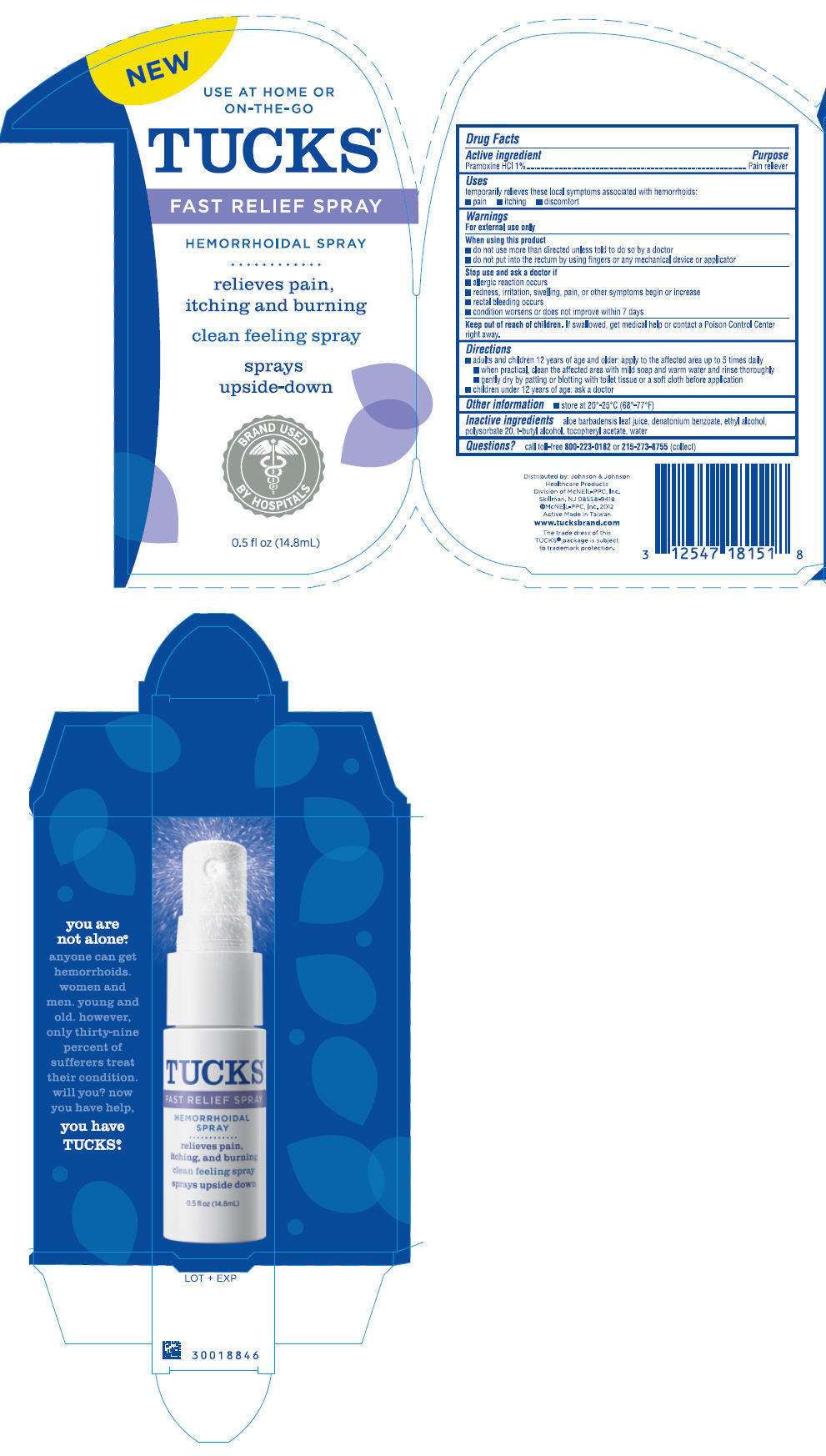
Drug Facts
Active ingredient
Pramoxine HCL 1%
Purpose
Pain reliever
Use
- temporary relieves these local symptoms associated with hemorrhoids:
- pain
- itching
- discomfort
Warnings
For external use only
When using this product
- do not use more than directed unless told to do so by a doctor
- do not put into the rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- allergic reaction occurs
- redness, irritation, swelling, pain, or other symptoms begin or increase
- rectal bleeding occurs
- condition worsens or does not improve within 7 days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and older: apply to the affected area up to 5 times daily
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before application
- children under 12 years of age: ask a doctor
Tucks Other information
- store at 20 to 25°C (68-77°F)
Inactive ingredients
aloe barbadensis leaf juice, denatonium benzoate, ethyl alcohol, polysorbate 20, t-butyl alcohol, tocopheryl acetate, water
Questions?
call toll-free 800-223-0182 or 215-273-8755 (collect)
Distributed by: Johnson & Johnson
Healthcare Products
Division of McNEIL-PPC, Inc.
Skillman, NJ 08558-9418
©McNEIL-PPC, Inc. 2012
PRINCIPAL DISPLAY PANEL - 14.8 mL Bottle Label
NEW
USE AT HOME OR
ON=THE-GO
TUCKS®
FAST RELIEF SPRAY
HEMORRHOIDAL SPRAY
Relieves pain,
itching and burning
clean feeling spray
sprays
upside-down
BRAND USED
BY HOSPITALS
0.5 fl oz (14.8mL)
TucksPramoxine Hydrochloride SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||