Ultram
FULL PRESCRIBING INFORMATION: CONTENTS*
- ULTRAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- ULTRAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ULTRAM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
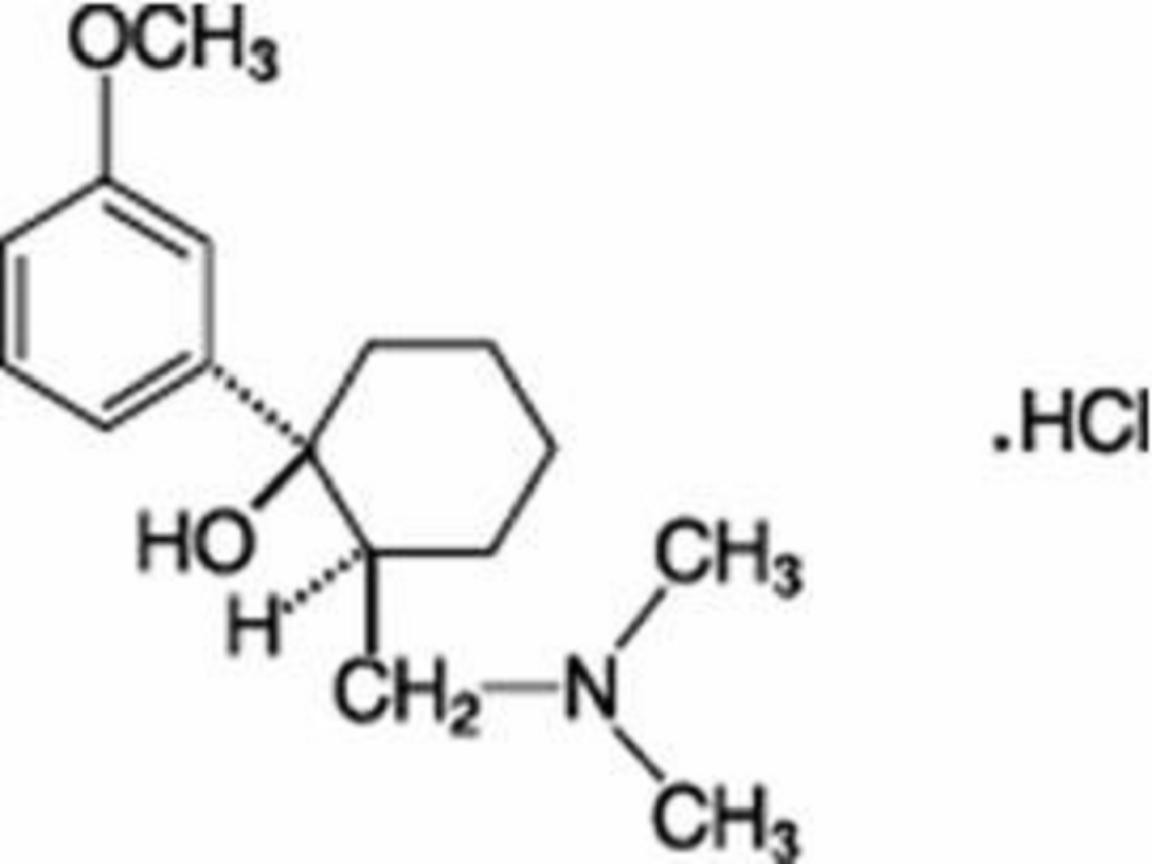
ULTRAM DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics
Absorption
Table 1Figure 2
Mean (%CV) Steady-State Pharmacokinetic Parameter Values (n=32)TramadolM1 MetabolitePharma- cokinetic ParameterULTRAM ER 200-mg Tablet Once-DailyULTRAM 50-mg Tablet Every 6 HoursULTRAM ER 200-mg Tablet Once-DailyULTRAM 50-mg Tablet Every 6 Hours
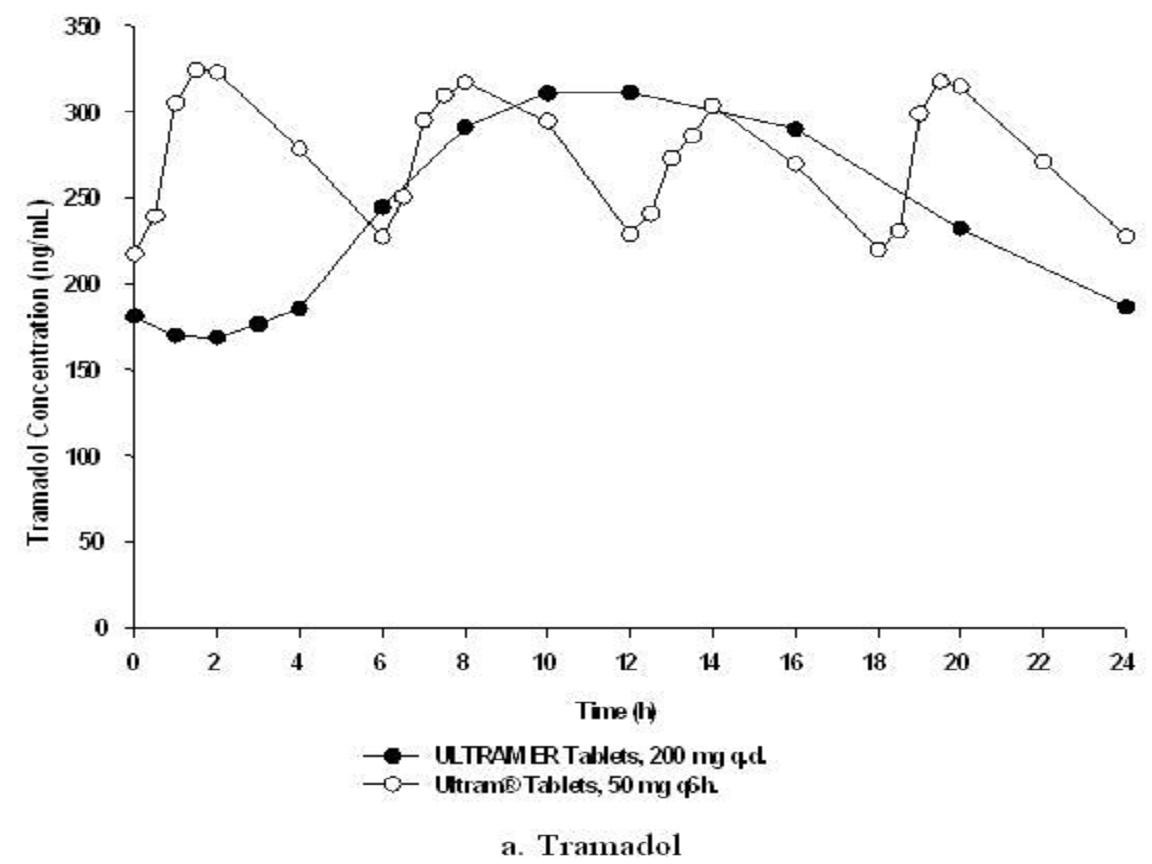
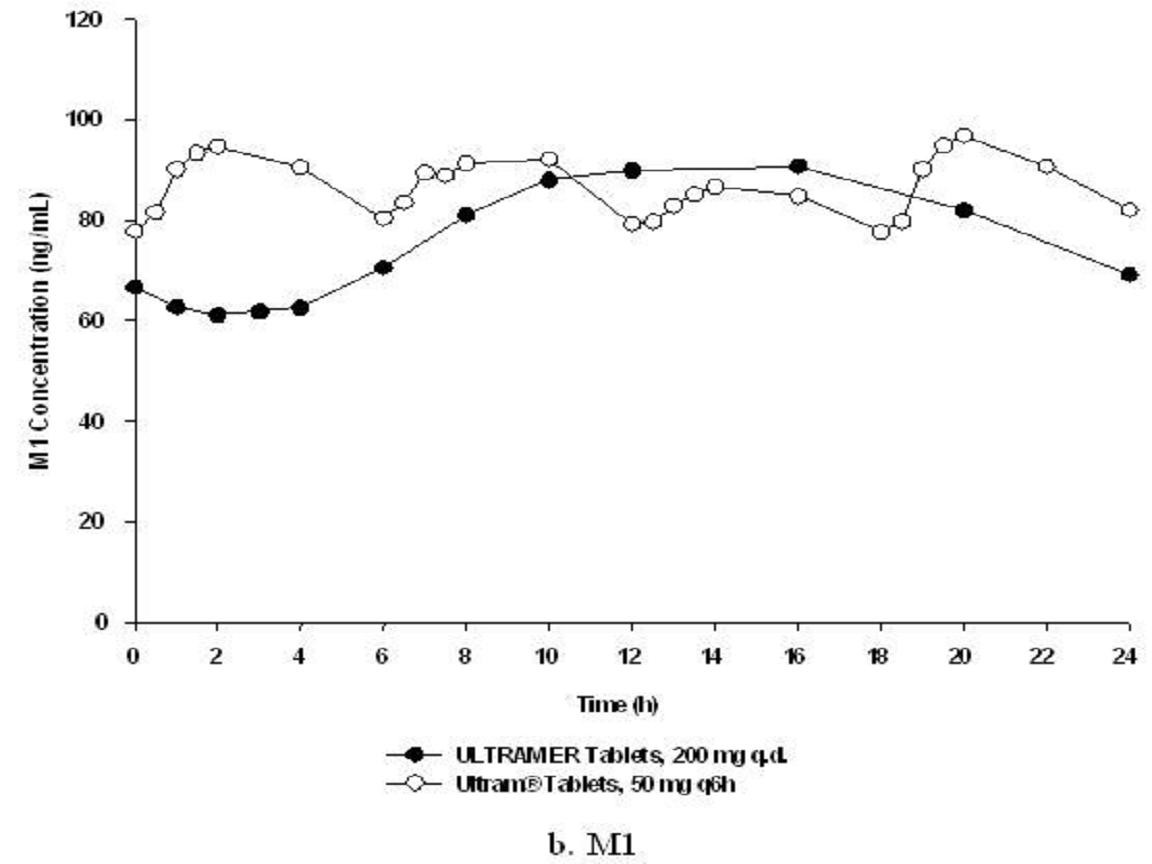
Food Effects
Distribution
Metabolism
PRECAUTIONS, Drug Interactions
Elimination
Special Populations
Renal
PRECAUTIONS, Use in Renal and Hepatic DiseaseDOSAGE AND ADMINISTRATION
Hepatic
PRECAUTIONS, Use in Renal and Hepatic DiseaseDOSAGE AND ADMINISTRATION
Geriatric
PRECAUTIONSDOSAGE AND ADMINISTRATION
Gender
Drug Interactions
PRECAUTIONS, Drug Interactions
Quinidine
PRECAUTIONS, Drug Interactions
Carbamazepine
PRECAUTIONS, Drug Interactions
Cimetidine
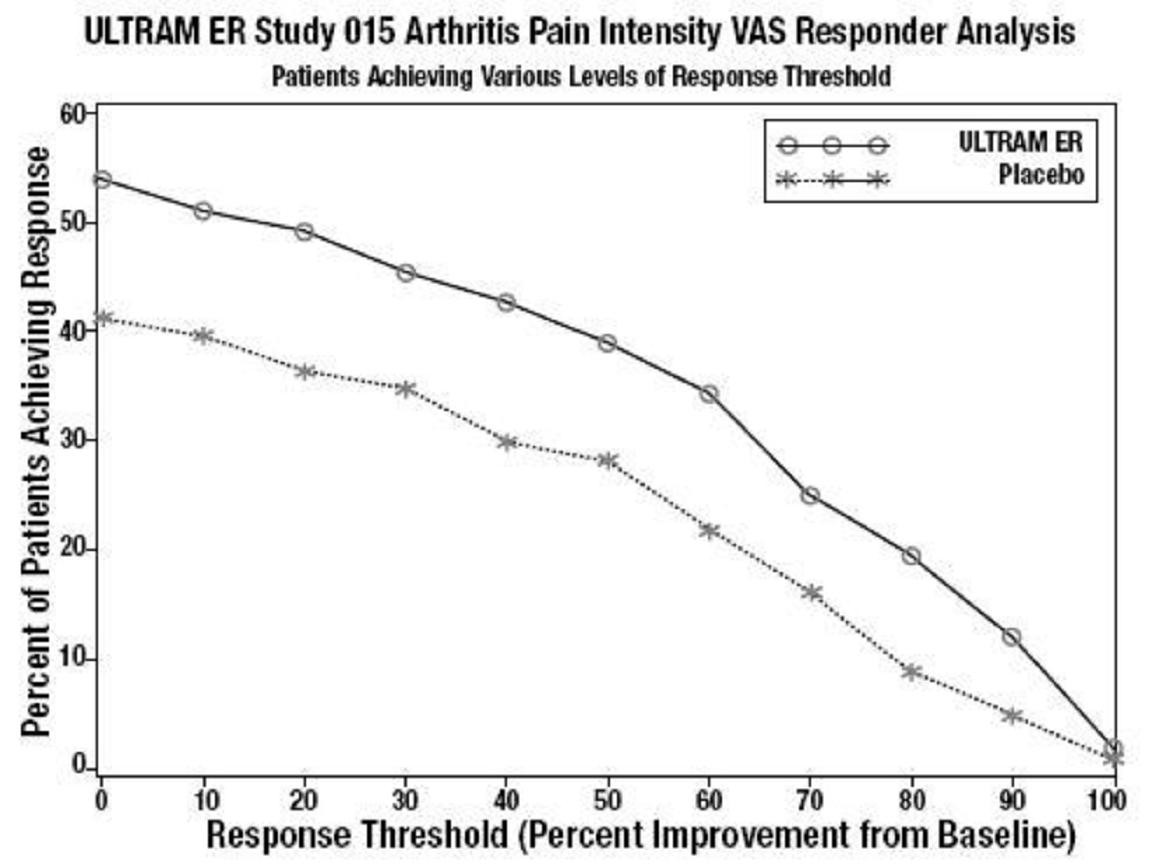
CLINICAL STUDIES
Figure 3
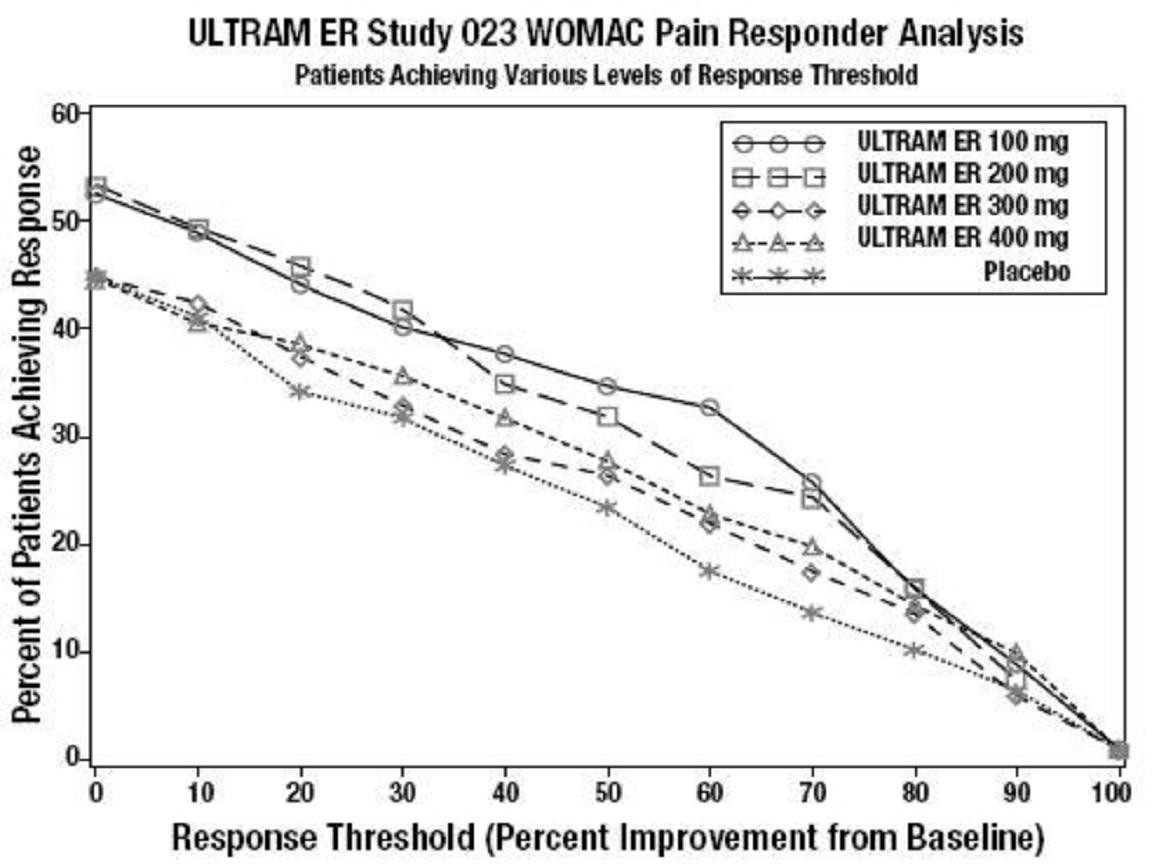
Figure 4
INDICATIONS & USAGE
ULTRAM CONTRAINDICATIONS
WARNINGS
Seizure RiskSeizures have been reported in patients receiving tramadol within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol above the recommended range. Concomitant use of tramadol increases the seizure risk in patients taking:
-
● Selective serotonin re-uptake inhibitors (SSRI antidepressants or anorectics),
-
● Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), or
-
● Other opioids.
-
● MAO inhibitors (see alsoWARNINGS, Use with MAO Inhibitors and Serotonin Re-uptake Inhibitors),
-
● Neuroleptics, or
-
● Other drugs that reduce the seizure threshold.
-
● Do not prescribe ULTRAM ER for patients who are suicidal or addiction-prone.
-
● Prescribe ULTRAM ER with caution for patients taking tranquilizers or antidepressant drugs and patients who use alcohol in excess.
-
● Tell your patients not to exceed the recommended dose and to limit their intake of alcohol.
Anaphylactoid Reactions
CONTRAINDICATIONS
Respiratory Depression
WARNINGS, Seizure RiskOVERDOSAGE
Interaction With Central Nervous System (CNS) Depressants
Increased Intracranial Pressure or Head Trauma
WARNINGS, Respiratory Depression
Use in Ambulatory Patients
Use With MAO Inhibitors and Serotonin Re-uptake Inhibitors
Withdrawal
Misuse, Abuse and Diversion of Opioids
WARNINGSDRUG ABUSE AND ADDICTION
Interactions with Alcohol and Drugs of Abuse
DRUG ABUSE AND ADDICTION
ULTRAMER is a mu-agonist opioid. Tramadol, like other opioids used in analgesia, can be abused and is subject to criminal diversion.
Risk of Overdosage
OVERDOSAGE
PRECAUTIONS
Acute Abdominal ConditionUse in Renal and Hepatic Disease
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONCLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
-
● Patients should be informed that ULTRAM ER is for oral use only and should be swallowed whole. The tablets should not be chewed, crushed, or split.
-
● Patients should be informed that ULTRAM ER may cause seizures and/or serotonin syndrome with concomitant use of serotonergic agents (including SSRIs, SNRIs, and triptans) or drugs that significantly reduce the metabolic clearance of tramadol.
-
● Patients should be informed that ULTRAM ER may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
-
● Patients should be informed that ULTRAM ER should not be taken with alcohol containing beverages.
-
● Patients should be informed that ULTRAM ER should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
-
● Female patients should be instructed to inform the prescriber if they are pregnant, think they might become pregnant, or are trying to become pregnant (seePRECAUTIONS, Labor and Delivery).
-
● Patients should be educated regarding the single-dose and 24-hour dosing regimen, as exceeding these recommendations can result in respiratory depression, seizures or death.
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY, Pharmacokinetics
WARNINGS, Serotonin Syndrome Risk
WARNINGS, Serotonin Syndrome Risk
Use With Carbamazepine
Use With Quinidine
CLINICAL PHARMACOLOGY, Drug Interactions
Use With Digoxin and Warfarin
Potential for Other Drugs to Affect Tramadol
Potential for Tramadol to Affect Other Drugs
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects: Pregnancy Category CNon-teratogenic Effects
LABOR & DELIVERY
DRUG ABUSE AND ADDICTIONNURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONULTRAM ADVERSE REACTIONS
Table 2ULTRAM ERPlaceboMedDRA Preferred Term100 mg (N=403) n (%)200 mg (N=400) n (%)300 mg (N=400) n (%)400 mg (N=202) n (%)(N=406) n (%)
Adverse events with incidence rates of 1.0% to <5.0%
Adverse events with incidence rates of 0.5% to <1.0% and serious adverse events reported in at least 2 patients.
OVERDOSAGE
DOSAGE & ADMINISTRATION
-
● creatinine clearance less than 30 mL/min,
-
● severe hepatic impairment (Child-Pugh Class C)
WARNINGS, Misuse, Abuse and Diversion of OpioidsDRUG ABUSE AND ADDICTION
Adults (18 years of age and over)
Patients Not Currently on Tramadol Immediate-Release Products
Patients Currently on Tramadol Immediate-Release Products
WARNINGS
Individualization of Dose
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

UltramTramadol Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!