Umecta
Umecta Nail Film
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Pharmacokinetics
- Indications and Umecta Uses
- Contraindications
- Warnings
- Precautions
- Pregnancy Category C
- Nursing Mothers
- Side Effects
- Dosage and Administration
- How Supplied
FULL PRESCRIBING INFORMATION
Description
Rx only
For topical use only
Not for ophthalmic use
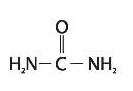
Clinical Pharmacology
Pharmacokinetics
The mechanism of action of topically applied urea is not yet known.
Indications and Uses
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful
for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns, and calluses, as well as damaged, ingrown and devitalized nails.
Contraindications
Warnings
For external use only. Avoid contact with eyes, lips or mucous membranes.
Precautions
Pregnancy Category C
Nursing Mothers
It is not known whether or not this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when Umecta is administered to a nursing woman.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Side Effects
Transient stinging, burning, itching, or irritation may occur and normally disappear on discontinuing the medication.
Dosage and Administration
How Supplied
Manufactured for:
Innocutis Holdings LLC
Charleston, SC 29401
Toll Free: 1-800-499-4468
www.innocutis.com
www.umecta.com


UmectaUrea FILM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||