Urimar-T
URIMAR-T™
FULL PRESCRIBING INFORMATION: CONTENTS*
- URIMAR-T DESCRIPTION
- CLINICAL PHARMACOLOGY
- URIMAR-T INDICATIONS AND USAGE
- URIMAR-T CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- URIMAR-T ADVERSE REACTIONS
- DRUG INTERACTIONS
- OVERDOSAGE
- URIMAR-T DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
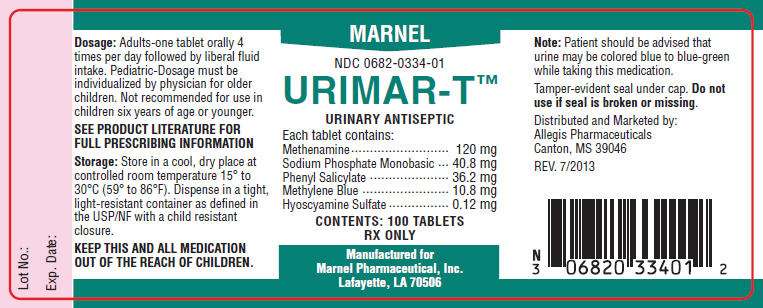
- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
FULL PRESCRIBING INFORMATION
Rx Only
URIMAR-T DESCRIPTION
URIMAR-T™ for oral administration.
| Each tablet contains: | |
|---|---|
| Inactive ingredients: colloidal silicon dioxide, lactose, polyethylene glycol, crospovidone polyplasdone, magnesium stearate, FD&C Lake Blue #1. | |
| Methenamine | 120 mg |
| Sodium Phosphate Monobasic | 40.8 mg |
| Phenyl Salicylate | 36.2 mg |
| Methylene Blue | 10.8 mg |
| Hyoscyamine Sulfate | 0.12 mg |
METHENAMINE
[100-97-0] 1,3,5,7-Tetraazatricyclo [3.3.1.-1 3,7] decane; hexamethylenetetramine; HMT; HMTA; hexamine; 1,3,5,7-tetraazaadamantane hexamethylenemine; Uritone; Urotropin. C6H12N4; mol wt 140.19; C 51.40%, H 8.63%, N 39.96%. Methenamine (hexamethylenetetramine) exists as colorless, lustrous crystals or white crystalline powder. Its solutions are alkaline to litmus. Freely soluble in water, soluble in alcohol and in chloroform.
SODIUM PHOSPHATE MONOBASIC
[7558-80-7] Phosphoric acid sodium salt (1:1); Sodium biphosphate; sodium dihydrogen phosphate; acid sodium phosphate; monosodium orthophosphate; primary sodium phosphate; H2NaO4P; mol wt 119.98, H 1.68%, Na 19.16%, O 53.34%, P 25.82%. Monohydrate, white, odorless slightly deliquesce crystals or granules. At 100° C loses all its water; when ignited it converts to metaphosphate. It is freely soluble in water and practically insoluble in alcohol. The aqueous solution is acid. pH of 0.1 molar aqueous solution at 25° C: 4.5.
PHENYL SALICYLATE
[118-55-8] 2-Hydroxybenzoic acid phenyl ester; Salol. C13H10O3; mol wt 214.22, C 72.89%, H 4.71%, O 22.41%. Made by the action of phosphorus oxy-chloride on a mixture of phenol and salicylic acid. Phenyl Salicylate exists as white crystals with a melting point of 41°-43° C. It is very slightly soluble in water and freely soluble in alcohol.
METHYLENE BLUE
[61-73-4] 3,7-Bis(dimethylamino) phenothiazin-5-ium chloride; C.I. Basic Blue 9; methylthioninium chloride; tetramethylthionine chloride; 3,7-bis(dimethylamino) phenazathionium chloride. C16H18ClN3S; mol wt 319.85, C 60.08%, H 5.67%, Cl 11.08%, N 13.14%, S 10.03%. Methylene Blue (Methylthionine chloride) exists as dark green crystals. It is soluble in water and in chloroform; sparingly soluble in alcohol.
HYOSCYAMINE SULFATE
[620-61-1] [3(S)-endo]-α-(Hydroxymethyl)-benzeneacetic acid 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester sulfate(2:1)(salt); 1αH,5αH-tropan-3α-ol(-)-tropate (ester) sulfate(2:1)(salt); 3α-tropanyl S-(-)-tropate; I-tropic acid ester with tropine; I-tropine tropate. C34H48N2O10S. Hyoscyamine Sulfate is an alkaloid of belladonna. Exists as a white crystalline powder. Its solutions are alkaline to litmus. Affected by light, it is slightly soluble in water; freely soluble in alcohol; sparingly soluble in ether.
CLINICAL PHARMACOLOGY
METHENAMINE degrades in an acidic urine environment releasing formaldehyde which provides bactericidal or bacteriostatic action. It is well absorbed from the gastrointestinal tract. 70 to 90% reaches the urine unchanged at which point it is hydrolyzed if the urine is acidic. Within 24 hours it is almost completely (90%) excreted; of this amount at pH 5, approximately 20% is formaldehyde. Protein binding - some formaldehyde is bound to substances in the urine and surrounding tissues. Methenamine is freely distributed to body tissue and fluids but is not clinically significant as it does not hydrolyze at a pH greater than 6.8.
SODIUM PHOSPHATE MONOBASIC an acidifier, helps to maintain an acid pH in the urine necessary for the degradation of methenamine.
PHENYL SALICYLATE releases salicylate, a mild analgesic for pain.
METHYLENE BLUE possesses weak antiseptic properties. It is well absorbed by the gastrointestinal tract and is rapidly reduced to leukomethylene blue which is stabilized in some combination form in the urine. 75% is excreted unchanged.
HYOSCYAMINE SULFATE is a parasympatholytic drug which relaxes smooth muscles and thus produces an antispasmotic effect . It is well absorbed from the gastrointestinal tract and is rapidly distributed throughout the body tissues. Most is excreted in the urine within 12 hours, 13% to 50% being unchanged. Protein binding for hyoscyamine sulfate is moderate and biotransformation is hepatic.
URIMAR-T INDICATIONS AND USAGE
URIMAR-T™ is indicated for the treatment of symptoms of irritative voiding. Indicated for the relief of local symptoms, such as inflammation, hypermotility, and pain, which accompany lower urinary tract infections. Indicated for the relief of urinary tract symptoms caused by diagnostic procedures.
URIMAR-T CONTRAINDICATIONS
Urimar-T™ is contraindicated in patients with a hypersensitivity to any of the ingredients. Risk-benefit should be considered when the following medical problems exist: Cardiac disease (especially cardiac arrhythmias, congestive heart failure, coronary heart disease, and mitral stenosis); gastrointestinal tract obstructive disease; glaucoma; myasthenia gravis; acute urinary retention may be precipitated in obstructive uropathy (such as bladder neck obstruction due to prostatic hypertrophy).
WARNINGS
Do not exceed recommended dosage. If rapid pulse, dizziness, or blurring of vision occurs, discontinue use immediately.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
PRECAUTIONS
Cross sensitivity and/or related problems
Patients intolerant of other belladonna alkaloids or other salicylates may be intolerant of this medication also. Delay in gastric emptying could complicate the management of gastric ulcers.
Pregnancy
Reproduction (FDA Pregnancy Category C)
Hyoscyamine and methenamine cross the placenta. Studies have not been done in either animals or humans. It is not known whether URIMAR-T™ tablets can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. URIMAR-T™ tablets should be given to a pregnant woman only if clearly needed.
Nursing mothers
Methenamine and traces of hyoscyamine are excreted in breast milk. Caution should be exercised when URIMAR-T™ tablets are administered to a nursing mother.
Prolonged use
There have been no studies to establish the safety of prolonged use in humans. No known long-term animal studies have been performed to evaluate carcinogenic potential.
Pediatric
Infants and young children are especially susceptible to the toxic effect of the belladonna alkaloids.
Geriatric
Use with caution in elderly patients as they may respond to the usual doses of the belladonna alkaloids with excitement, agitation, drowsiness, or confusion.
URIMAR-T ADVERSE REACTIONS
Cardiovascular - rapid pulse, flushing
Central Nervous System - blurred vision, dizziness, drowsiness
Respiratory - shortness of breath or troubled breathing
Genitourinary - difficult micturition, acute urinary retention
Gastrointestinal -dry mouth, nausea and vomiting
Serious allergic reactions to this drug are rare. Seek immediate medical attention if you notice symptoms of a serious allergic reaction, including itching, rash, severe dizziness, swelling or trouble breathing.
This medication can cause urine and sometimes stools to turn blue to blue-green. This effect is harmless and will subside after medication is stopped.
Call your doctor or physician for medical advice about side effects. To report SUSPECTED ADVERSE REACTIONS, contact Marnel Pharmaceutical, Inc. at 337-232-1396, or FDA at 1-800-FDA-1088, www.fda.gov/medwatch.
DRUG INTERACTIONS
As a result of hyoscyamine's effects on gastrointestinal motility and gastric emptying, absorption of other oral medications may be decreased during concurrent use with this combination medication.
Urinary alkalizers and thiazide diuretics
May cause the urine to become alkaline reducing the effectiveness of methenamine by inhibiting its conversion to formaldehyde.
Antimuscarinics
Concurrent use may intensify antimuscarinic effects of hyoscyamine because of secondary antimuscarinic activities of these medications.
Antacids/antidiarrheals
Concurrent use may reduce absorption of hyoscyamine resulting in decreased therapeutic effectiveness. Concurrent use with antacids may cause urine to become alkaline reducing the effectiveness of methenamine by inhibiting its conversion to formaldehyde. Doses of these medications should be spaced 1 hour apart from doses of hyoscyamine.
Antimyasthenics
Concurrent use with hyoscyamine may further reduce intestinal motility, therefore, caution is recommended.
Ketoconazole and hyoscyamine may cause increased gastrointestinal pH.
Concurrent administration with hyoscyamine may result in marked reduction in the absorption of ketoconazole. Patients should be advised to take this combination at least 2 hours after ketoconazole.
Monoamine oxidase (MAO) inhibitors
Concurrent use with hyoscyamine may intensify antimuscarinic side effects.
Opioid (narcotic) analgesics may result in increased risk of severe constipation.
Sulfonamides
These drugs may precipitate with formaldehyde in the urine increasing the danger of crystalluria.
Patients should be advised that the urine and/or stools may become blue to blue-green as a result of the excretion of methylene blue.
Drug Abuse And Dependence
A dependence on the use of URIMAR-T™ has not been reported and due to the nature of its ingredients, abuse of URIMAR T™ is not expected.
OVERDOSAGE
Emesis or gastric lavage. Slow intravenous administration of physostigmine in doses of 1 to 4 mg (0.5 to 1 mg in children) repeated as needed in one to two hours to reverse severe antimuscarinic symptoms.
Administration of small doses of diazepam to control excitement and seizures.
Artificial respiration with oxygen if needed for respiratory depression.
Adequate hydration.
Symptomatic treatment as necessary.
If overdose is suspected, contact the poison control center at 1-800-222-1222, or your local emergency room immediately.
URIMAR-T DOSAGE AND ADMINISTRATION
Adults
One tablet orally 4 times per day followed by liberal fluid intake.
Pediatric
Dosage must be individualized by physician for older children. Not recommended for use in children six years of age or younger.
HOW SUPPLIED
Urimar-T™ are blue tablets imprinted with 334 on one side and plain on the other, available in bottles of 100 tablets, (NDC: 0682-0334-01), and sample tablets of 1, (NDC: 0682-0334-10).
STORAGE
Store in a cool, dry place at controlled room temperature 15° to 30°C (59° to 86°F). Keep container tightly closed. Protect from moisture and direct sunlight.
Dispense in a tight, light-resistant container as defined in the USP/NF with a child resistant closure.
WARNING: Keep this and all drugs out of reach of children.
Rx Only
Manufactured for:
Marnel Pharmaceutical, Inc.
Lafayette, LA 70506
Distributed and Marketed by:
Allegis Pharmaceuticals
Canton, MS 39046
Rev. 7/2013
URIMAR-T™ is a registered trademark of Marnel Pharmaceutical, Inc.
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
MARNEL
NDC 0682-0334-01
URIMAR-T™
URINARY ANTISEPTIC
Each tablet contains:
Methenamine 120 mg
Sodium Phosphate Monobasic 40.8 mg
Phenyl Salicylate 36.2 mg
Methylene Blue 10.8 mg
Hyoscyamine Sulfate 0.12 mg
CONTENTS: 100 TABLETS
RX ONLY
Manufactured for
Marnel Pharmaceutical, Inc.
Lafayette, LA 70506
Urimar-TMethenamine, Sodium Phosphate, Monobasic, Monohydrate, Phenyl Salicylate, Methylene Blue, and Hyoscyamine Sulfate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||