Valium
FULL PRESCRIBING INFORMATION: CONTENTS*
- VALIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- VALIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- VALIUM ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
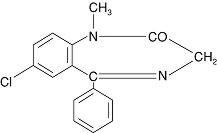
VALIUM DESCRIPTION
CLINICAL PHARMACOLOGY
Pharmacokinetics
Pharmacokinetics in Special Populations
INDICATIONS
VALIUM CONTRAINDICATIONS
WARNINGS
Pregnancy
Labor and Delivery
Nursing Mothers
PRECAUTIONS
GeneralDrug Interactions).
ADVERSE REACTIONS). Should this occur, use of the drug should be discontinued. These reactions are more likely to occur in children and the elderly.
DRUG ABUSE AND DEPENDENCE).
Information For Patients
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Category D(seeWARNINGS: Pregnancy).
Pediatric Use
Geriatric Use
Hepatic Insufficiency
CLINICAL PHARMACOLOGY: Pharmacokinetics in Special Populations: Hepatic Insufficiency).
VALIUM ADVERSE REACTIONS
Central Nervous System:
Gastrointestinal System:
Special Senses:
Cardiovascular System:
Psychiatric and Paradoxical Reactions:
Urogenital System:
Skin and Appendages:
Laboratories:
Other:
DRUG ABUSE AND DEPENDENCE
Rebound Anxiety:
OVERDOSAGE
Management of Overdosage
The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.Caution should be observed in the use of flumazenil in epileptic patients treated with benzodiazepines. The complete flumazenil package insert, includingCONTRAINDICATIONS, WARNINGS,andPRECAUTIONS,should be consulted prior to use.
DRUG ABUSE AND DEPENDENCE).
DOSAGE & ADMINISTRATION
ADULTS: USUAL DAILY DOSE:
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Valiumdiazepam TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!