Velphoro
Fresenius Medical Care North America
Vifor Fresenius Medical Care Renal Pharma France
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGE Velphoro is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis. (1) DOSAGE AND ADMINISTRATION Velphoro tablets must be chewed and not swallowed whole. To aid with chewing and swallowing, tablets may be crushed. (2) The recommended starting dose of Velphoro is 3 tablets (1,500 mg) per day, administered as 1 tablet (500 mg) 3 times daily with meals. (2) Adjust by 1 tablet per day as needed until an acceptable serum phosphorus level (less than or equal to 5.5 mg/dL) is reached, with regular monitoring afterwards. Titrate as often as weekly. (2) DOSAGE FORMS AND STRENGTHS Velphoro (sucroferric oxyhydroxide) chewable tablet 500 mg (3) CONTRAINDICATIONS None. WARNINGS AND PRECAUTIONS Patients with peritonitis during peritoneal dialysis, significant gastric or hepatic disorders, following major gastrointestinal surgery, or with a history of hemochromatosis or other diseases with iron accumulation have not been included in clinical studies with Velphoro. Monitor effect and iron homeostasis in such patients. (5.1) Side Effects In a parallel design, fixed-dose study of 6 weeks duration, the most common adverse drug reactions to Velphoro chewable tablets in hemodialysis patients included discolored feces (12%) and diarrhea (6%). (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Medical Care North America at 1-800-323-5188 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Velphoro can be administered concomitantly with ciprofloxacin, digoxin, enalapril, furosemide, HMG-CoA reductase inhibitors, hydrochlorothiazide, losartan, metformin, metoprolol, nifedipine, omeprazole, quinidine and warfarin. (7) Take alendronate and doxycycline at least 1 hour before Velphoro. (7) Velphoro should not be prescribed with oral levothyroxine and oral vitamin D analogs. (7)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 VELPHORO INDICATIONS AND USAGE
- 2 VELPHORO DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 VELPHORO CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 VELPHORO ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 VELPHORO DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Velphoro (sucroferric oxyhydroxide) is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis.
2 DOSAGE AND ADMINISTRATION
Velphoro tablets must be chewed and not swallowed whole. To aid with chewing and swallowing, the tablets may be crushed.
Starting Dose
The recommended starting dose of Velphoro is 3 tablets (1,500 mg) per day, administered as 1 tablet (500 mg) 3 times daily with meals.
Titration and Maintenance
Serum phosphorus levels should be monitored and the dose of Velphoro titrated in decrements or increments of 500 mg (1 tablet) per day as needed until an acceptable serum phosphorus level (less than or equal to 5.5 mg/dL) is reached, with regular monitoring afterwards. Titration can be started as early as 1 week after treatment initiation and adjusted at weekly intervals thereafter if necessary.
Based on clinical studies, on average patients required 3 to 4 tablets (1,500 mg to 2,000 mg) a day to control serum phosphorus levels.
The highest daily dose studied in a Phase 3 clinical trial in ESRD patients was 6 tablets (3,000 mg) per day.
Administration
Velphoro must be administered with meals. To maximize the dietary phosphate binding, the total daily dose should be divided across the meals of the day. No additional fluid above the amount usually taken by the patient is required.
If one or more doses of Velphoro are missed, the medication should be resumed with the next meal. Do not attempt to replace a missed dose.
3 DOSAGE FORMS AND STRENGTHS
Velphoro (sucroferric oxyhydroxide) chewable tablet 500 mg.
Each chewable tablet contains 500 mg iron (equivalent to 2,500 mg sucroferric oxyhydroxide).
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Monitoring in Patients with Gastrointestinal Disorders or Iron Accumulation Disorders
Patients with peritonitis during peritoneal dialysis, significant gastric or hepatic disorders, following major gastrointestinal (GI) surgery, or with a history of hemochromatosis or other diseases with iron accumulation have not been included in clinical studies with Velphoro. Monitor effect and iron homeostasis in such patients.
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data derived from Velphoro clinical trials reflect exposure to Velphoro in 2 active-controlled clinical studies involving a total of 778 patients on hemodialysis and 57 patients on peritoneal dialysis exposed for up to 55 weeks. Dosage regimens ranged from 250 mg to 3,000 mg per day.
As expected with oral preparations containing iron, discolored (dark colored) feces was a commonly occurring adverse drug reaction.
In a parallel design, dose-finding study of Velphoro with a treatment duration of 6 weeks in hemodialysis patients, adverse reactions for Velphoro (N=128) were similar to those reported for the active-control group (sevelamer hydrochloride) (N=26), with the exception of discolored feces (12%) which did not occur in the active-control group and diarrhea (6%).
In a 55-week, open-label, active-controlled, parallel design, safety and efficacy study involving 968 hemodialysis patients and 86 peritoneal dialysis patients treated with either Velphoro (N=707 including 57 peritoneal dialysis patients) or the active-control (sevelamer carbonate) (N=348 including 29 peritoneal dialysis patients), adverse reactions occurring more than 5% in the Velphoro group were diarrhea (24%), discolored feces (16%), and nausea (10%). The majority of diarrhea events in the Velphoro group were mild and transient, occurring soon after initiation of treatment, and resolving with continued treatment. Similar adverse reactions occurred at similar rates in hemodialysis and peritoneal dialysis patients. The most common adverse reactions (>1%) leading to withdrawal were diarrhea (4%), product taste abnormal (2%), and nausea (2%).
7 DRUG INTERACTIONS
|
Drugs that can be administered concomitantly with Velphoro |
|
| Ciprofloxacin Digoxin Enalapril Furosemide HMG-CoA reductase inhibitors Hydrochlorothiazide Losartan Metformin Metoprolol Nifedipine Omeprazole Quinidine Warfarin |
|
|
Drugs that are to be separated from Velphoro and meals |
|
| Dosing Recommendations | |
| Alendronate Doxycycline |
Take these at least 1 hour before Velphoro. |
|
Oral drugs that should not be prescribed with Velphoro |
|
| Levothyroxine Vitamin D analogs |
|
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B: Reproduction studies have been performed in rats and rabbits at doses up to 16 and 4 times, respectively, the human maximum recommended clinical dose on a body weight basis, and have not revealed evidence of impaired fertility or harm to the fetus due to Velphoro [see Nonclinical Toxicology (13.2)]. However, Velphoro at a dose up to 16 times the maximum clinical dose was associated with an increase in post-implantation loss in pregnant rats. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
There are no adequate and well-controlled studies in pregnant women.
8.2 Labor and Delivery
No Velphoro treatment-related effects on labor and delivery were seen in animal studies with doses up to 16 times the maximum recommended clinical dose on a body weight basis. The effects of Velphoro on labor and delivery in humans are not known.
8.3 Nursing Mothers
Since the absorption of iron from Velphoro is minimal [see Clinical Pharmacology (12.3)], excretion of Velphoro in breast milk is unlikely.
8.4 Pediatric Use
The safety and efficacy of Velphoro have not been established in pediatric patients.
8.5 Geriatric Use
Of the total number of subjects in two active-controlled clinical studies of Velphoro (N=835), 29.7% (n=248) were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
10 OVERDOSAGE
There are no reports of overdosage with Velphoro in patients. Since the absorption of iron from Velphoro is low [see Clinical Pharmacology (12.3)], the risk of systemic iron toxicity is negligible. Hypophosphatemia should be treated by standard clinical practice.
Velphoro has been studied in doses up to 3,000 mg per day.
11 DESCRIPTION
Velphoro chewable tablets are brown, circular, bi-planar, and are embossed with “PA 500” on 1 side. Each tablet of Velphoro contains 500 mg iron (in 2,500 mg sucroferric oxyhydroxide). The Velphoro drug substance is a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches. The active moiety, polynuclear iron(III)-oxyhydroxide, is practically insoluble and cannot be absorbed. The inactive ingredients are woodberry flavor, neohesperidin dihydrochalcone, magnesium stearate, and silica (colloidal, anhydrous).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In the aqueous environment of the GI tract, phosphate binding takes place by ligand exchange between hydroxyl groups and/or water in sucroferric oxyhydroxide and the phosphate in the diet. The bound phosphate is eliminated with feces.
Both serum phosphorus levels and calcium-phosphorus product levels are reduced as a consequence of the reduced dietary phosphate absorption.
12.2 Pharmacodynamics
In vitro studies have demonstrated a robust phosphate binding capacity of Velphoro over the physiologically relevant pH range of the GI tract (1.2-7.5). The phosphate binding capacity of Velphoro peaked at pH 2.5, resulting in 96% of the available phosphate being adsorbed (phosphorus:iron concentration ratio 0.4:1).
12.3 Pharmacokinetics
The active moiety of Velphoro, polynuclear iron(III)-oxyhydroxide (pn-FeOOH), is practically insoluble and therefore not absorbed and not metabolized. Its degradation product, mononuclear iron species, can however be released from the surface of pn-FeOOH and be absorbed.
Because of the insolubility and degradation characteristics of Velphoro, no classical pharmacokinetic studies can be carried out.
The sucrose and starch components of Velphoro can be digested to glucose and fructose, and maltose and glucose, respectively. These compounds can be absorbed in the blood. One tablet is equivalent to 1.4 g of carbohydrates.
The iron uptake from radiolabelled Velphoro drug substance, 2,000 mg in 1 day, was investigated in 16 chronic kidney disease patients (8 pre-dialysis and 8 hemodialysis patients) and 8 healthy volunteers with low iron stores (serum ferritin <100 mcg/L). In healthy subjects, the median uptake of radiolabelled iron in the blood was 0.43% on Day 21. In chronic kidney disease patients, the median uptake was much less, 0.04% on Day 21.
Drug Interaction Studies
In vitro
In vitro interactions were studied in aqueous solutions which mimic the physico-chemical conditions of the gastro-intestinal tract with or without the presence of phosphate (400 mg). The study was conducted at pH 3.0, 5.5 and 8.0 with incubation at 37°C for 6 hours.
Interaction with Velphoro was seen with the following drugs: alendronate, doxycycline, levothyroxine, and paricalcitol.
Following drugs did not show interaction with Velphoro: ciprofloxacin, enalapril, hydrochlorothiazide, metformin, metoprolol, nifedipine, and quinidine.
In vivo
Five in vivo drug interaction studies (N=40/study) were conducted with losartan, furosemide, digoxin, omeprazole and warfarin in healthy subjects receiving 1,000 mg Velphoro 3 times a day with meals. Velphoro did not alter the systemic exposure as measured by the area under the curve (AUC) of the tested drugs when co-administered with Velphoro or given 2 hours later.
Data from the clinical studies (Study-05A and Study-05B) show that Velphoro does not affect the lipid lowering effects of HMG-CoA reductase inhibitors.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were performed in mice and rats.
In the 2-year carcinogenicity study in mice, animals were given Velphoro by diet at doses of 250, 500 or 1,000 mg/kg/day. Rare but not statistically significant neoplastic adenocarcinomas were seen in the colon of male mice at doses of 500 and 1,000 mg/kg/day. For a 60 kg person, the no-observed-adverse-effect level (NOAEL) of 250 mg/kg/day represents 5 times (on a body weight basis) the maximum recommended clinical dose of 3,000 mg/day. In addition, an increased incidence of epithelial hyperplasia was seen in the colon at all dosage levels (i.e., ≥5 times the maximum recommended clinical dose) and in the cecum at the highest dosage (equivalent to 20 times the maximum recommended clinical dose). The development of adenocarcinoma in the male mice was considered not a genotoxic effect, but the result of chronic local irritation from high amounts of intraluminal Velphoro in the GI tract.
In the 2-year rat carcinogenicity study, animals were given Velphoro by diet at doses of 40, 150 or 500 mg/kg/day. No statistically significantly increased incidences of tumors were found, but there were increased incidences in epithelial hyperplasia with or without submucosal inflammation in duodenum, cecum and colon at the dose of 500 mg/kg/day (10 times the maximum recommended clinical dose).
Velphoro was not mutagenic, clastogenic or DNA damaging in vitro in the Ames bacterial reverse mutation test, or in the Chinese-hamster fibroblast chromosomal aberration test, or in vivo in the rat Comet assay or peripheral blood micronucleus test.
In rats, mating performance and fertility were unaffected by Velphoro at oral doses up to 800 mg/kg/day (16 times the maximum recommended clinical dose).
13.2 Animal Toxicity and/or Pharmacology
In pregnant rats given up to 800 mg/kg/day Velphoro by oral gavage from Days 6 to 17 post-mating, no embryo-fetal development toxicity was observed. This dose corresponds to 16 times the maximum recommended clinical dose.
In pregnant rabbits given 50, 100 or 200 mg/kg/day Velphoro by oral gavage, from Days 6 to 19 post-mating, the number of fetuses with incomplete/unossified epiphyses and metacarpals/phalanges was increased at the highest dose (corresponding to 4 times the recommended maximum clinical dose). Litter parameters were not adversely affected.
In pregnant rats given Velphoro at 100, 280, or 800 mg/kg/day by oral gavage from Day 6 post-mating to lactation Day 20, offspring body weight gain was lower at age 5-13 weeks and neuromuscular function was delayed at the dose of 800 mg/kg/day. This dose represented 16 times the maximum recommended clinical dose.
14 CLINICAL STUDIES
The ability of Velphoro to lower serum phosphorus in ESRD patients on dialysis was demonstrated in 2 randomized clinical trials: one 6-week, open-label, active-controlled (sevelamer hydrochloride), dose-finding study; and one 55-week, open-label, active-controlled (sevelamer carbonate), parallel-group, safety and efficacy study.
In clinical trials, control of serum phosphorus levels was demonstrated at doses starting from 1,000 mg (2 tablets) per day with treatment effect being observed as early as 1-2 weeks after starting Velphoro.
14.1 Fixed-dose Study
In Study-03A, 154 ESRD patients on hemodialysis who were hyperphosphatemic (serum phosphorus >5.5 mg/dL but <7.75 mg/dL) following a 2-week phosphate binder washout period, were randomized to receive Velphoro at 250 mg/day, 1,000 mg/day, 1,500 mg/day, 2,000 mg/day, or 2,500 mg/day or active-control (sevelamer hydrochloride). Velphoro treatment was divided across meals, depending on dose. No dose titration was allowed. Within each of the groups, the serum phosphorus level at the end of treatment was compared to baseline value. Velphoro was shown to be efficacious (p≤0.016) for all doses except 250 mg/day. There were no patient-reported dose limiting treatment-emergent adverse events.
Mean changes in iron parameters (ferritin, transferrin saturation (TSAT) and transferrin) and vitamins (A, D, E and K) were generally not clinically meaningful and showed no apparent trends across the treatment groups. Velphoro had a similar GI adverse event profile [see Adverse Reactions (6.1)] to sevelamer hydrochloride, and no dose-dependent trend in GI events was observed.
14.2 Dose Titration Study
In Study-05A, 1,054 patients on hemodialysis (N=968) or peritoneal dialysis (N=87) with serum phosphorus ≥6 mg/dL following a 2-4 week phosphate binder washout period, were randomized and treated with either Velphoro, at a starting dose of 1,000 mg/day (N=707), or active-control (sevelamer carbonate, N=348) for 24 weeks. At the end of Week 24, 93 patients on hemodialysis whose serum phosphorus levels were controlled (<5.5 mg/dL) with Velphoro in the first part of the study, were re-randomized to continue treatment with either their Week 24 maintenance dose (N=44 or a non-effective low dose control 250 mg/day, N=49) of Velphoro for a further 3 weeks. At Week 27, a superiority analysis of the Velphoro maintenance dose versus low dose was performed. The maximum dose of Velphoro was 3,000 mg/day (6 tablets/day) and the minimum dose was 1,000 mg/day (2 tablets/day). Velphoro was administered with food and the daily dose was divided across the largest meals of the day.
The Velphoro maintenance dose (1,000 to 3,000 mg/day) was statistically significantly superior in sustaining the phosphorus lowering effect in hemodialysis patients at Week 27 (p<0.001) compared with the non-effective low dose control. The results are provided in Table 1.
| * p<0.001 for the difference in least square means of the change from BL to Week 27/End of Treatment (LOCF principle) between Velphoro maintenance dose and low dose using a covariance analysis (MIXED Model). Notes: BL is Week 24 or latest value available before Week 24 when Week 24 result is missing; End of Treatment is Week 27 value or includes the latest evaluable measurement after Week 24 (i.e., LOCF). BL = Baseline; LOCF = Last observation carried forward; SD = Standard deviation. |
||
| Mean (SD) Serum Phosphorus (mg/dL) | ||
|
Velphoro Maintenance Dose (1,000 to 3,000 mg/day) (N=44) |
Velphoro Low Dose Control (250 mg/day) (N=49) |
|
| Week 24 (BL) | 4.7 (1.03) | 5.0 (1.14) |
| Week 25 | 4.7 (0.91) | 6.3 (1.44) |
| Week 26 | 4.7 (1.21) | 6.6 (1.91) |
|
Week 27/End of Treatment |
5.0 (1.07) | 6.8 (1.63) |
|
Change from BL to End of Treatment |
0.3 (1.22)* | 1.8 (1.47) |
Following completion of Study-05A, 658 patients (597 on hemodialysis and 61 on peritoneal dialysis) were treated in the 28-week extension study (Study-05B) with either Velphoro (N=391) or sevelamer carbonate (N=267) according to their original randomization.
Serum phosphorus levels declined rapidly during the first few weeks of treatment and remained relatively constant thereafter. The phosphorus lowering effect of Velphoro was consistently maintained through 12 months of treatment (shown in Figure1).Age, gender, race, or dialysis modality did not affect the efficacy of Velphoro.
Serum iron level increases from baseline were not clinically meaningful and did not differ significantly compared to the active control. There was no evidence of accumulation of iron during one year treatment.
There were no clinically meaningful changes for vitamins (A, D, E and K) with Velphoro.
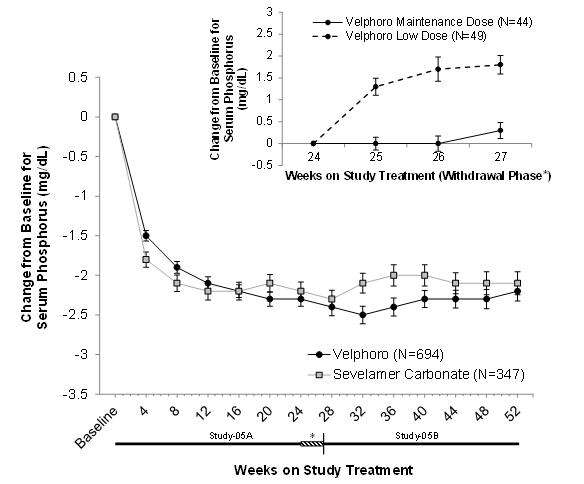
| Figure 1 | Mean change (±SEM) from baseline in serum phosphorus over time in Study-05A and extension Study-05B. Insert showing the mean change (±SEM) from baseline in serum phosphorus during the withdrawal phase of the study (Weeks 24 to 27) for Velphoro non-effective low dose control (250 mg/day) versus Velphoro maintenance dose. |
16 HOW SUPPLIED/STORAGE AND HANDLING
Velphoro are chewable tablets supplied as brown, circular, bi-planar tablets, embossed with "PA 500" on 1 side. Each tablet of Velphoro contains 500 mg iron as sucroferric oxyhydroxide. Velphoro tablets are packaged as follows:
NDC 49230-645-51 Bottle of 90 chewable tablets
Storage
Store in the original package and keep the bottle tightly closed in order to protect from moisture.
Store at 25°C (77°F) with excursions permitted to 15 to 30°C (59 to 86°F).
17 PATIENT COUNSELING INFORMATION
• Dosing Recommendations
Inform patients that Velphoro tablets must be chewed and not swallowed whole. To aid with chewing and swallowing, the tablets may be crushed [see Dosage and Administration (2)].
Velphoro should be taken with meals.
Some drugs need to be given at least one hour before Velphoro [see Drug Interactions. (7)]
• Adverse Reactions
Velphoro can cause discolored (black) stool. Discolored (black) stool may mask GI bleeding. Velphoro does not affect guaiac based (Hämocult) or immunological based (iColo Rectal, and Hexagon Opti) fecal occult blood tests.
Distributed by:
Fresenius Medical Care North America
920 Winter Street
Waltham, MA 02451
US Patent Nos. 6174442 and pending, comparable and/or related patents.
© 2013 Fresenius Medical Care North America. All rights reserved.
Principal Display Label - Bottle Label - NDC 49230-645-51

Principal Display Label - Bottle Carton - NDC 49230-645-51

Velphorosucroferric oxyhydroxide TABLET, CHEWABLE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||