Venlafaxine Hydrochloride
PD-Rx Pharmaceuticals, Inc.
PD-Rx Pharmaceuticals, Inc.
Venlafaxine Hydrochloride Extended-Release Capsules, USPRx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- VENLAFAXINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- VENLAFAXINE HYDROCHLORIDE INDICATIONS AND USAGE
- VENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- VENLAFAXINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of venlafaxine hydrochloride extended-release capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use)
VENLAFAXINE HYDROCHLORIDE DESCRIPTION
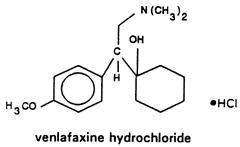
Venlafaxine hydrochloride extended-release capsules, USP for oral administration contain venlafaxine hydrochloride, a structurally novel antidepressant. It is designated (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[α- [(dimethylamino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride and has the empirical formula of C17H27NO2 HCl. Its molecular weight is 313.87. The structural formula is shown below.
CLINICAL PHARMACOLOGY
VENLAFAXINE HYDROCHLORIDE INDICATIONS AND USAGE
VENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
Hypersensitivity to venlafaxine hydrochloride or to any excipients in the formulation.
The use of MAOIs intended to treat psychiatric disorders with venlafaxine hydrochloride extended-release capsules or within 7 days of stopping treatment with venlafaxine hydrochloride extended-release capsules are contraindicated because of an increased risk of serotonin syndrome. The use of venlafaxine hydrochloride extended-release capsules within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated (see WARNINGS and DOSAGE AND ADMINISTRATION).
Starting venlafaxine hydrochloride extended-release capsules in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome (see WARNINGS and DOSAGE AND ADMINISTRATION).
WARNINGS
PRECAUTIONS
The 75 mg and 150 mg capsules contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Carcinogenesis, Mutagenesis, Impairment of Fertility
VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
VENLAFAXINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Venlafaxine Hydrochloride Extended-Release Capsules, 37.5 mg

Venlafaxine HydrochlorideVenlafaxine Hydrochloride CAPSULE, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||