Verucide Physician Formula
Blaine Labs Inc.
Blaine Labs Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Inactive Ingredients
- Purpose
- Verucide Physician Formula Uses
- Directions
- Keep Out of Reach of Children
- Warnings
- Ask A Doctor
- Package Label
FULL PRESCRIBING INFORMATION
Active Ingredients
Salicylic Acid 17%
Alcohol 60%
Inactive Ingredients
Water, SDA Alcohol 40B, Carcia Papaya Fruit Extract, Hydroxypropyl-cellulose, Artemisia Scoparia Oil, FD C Brown 1
Purpose
Wart Removal
Skin Cleanser
Verucide Physician Formula Uses
For the removal of common warts (easily recognized by the rough cauliflower-like appearance of the surface) For the removal of plantar warts that are located on the bottom of the foot.
Directions
Wash Affected area with one or two drops of the Pre-Treatment Solution and scrub with a brush. Rinse and dry thoroughly. Dab the wart removal solution directly onto the wart and allow to dry. Repeat steps once daily until the wart is gone.
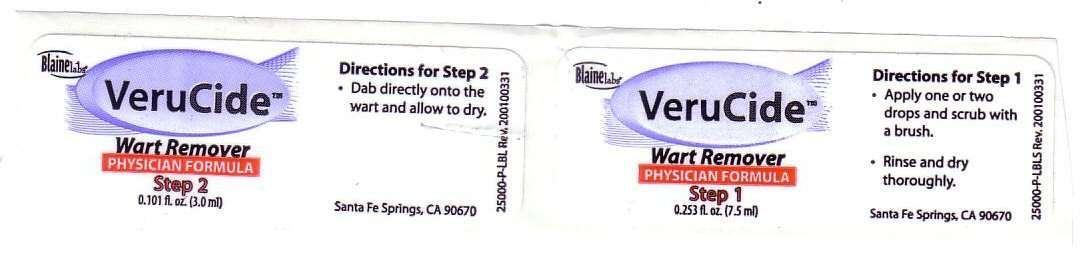
Directions for Step 1 Apply one or two drops and scrub with a brush Rinse and dry thoroughly
Directions for Step 2 Dab directly onto the wart and allow to dry.
Keep Out of Reach of Children
Keep Out Of Reach Of Children
Warnings
Avoid contact with the eyes. If eye contact does occur, rinse thoroughly with water for 15 minutes. For external use only. Avoid inhaling vapors. Extremely flammable. Keep away from fire or flame. Replace bottle caps tightly and store at room temperature away from heat. Do not use on irritated skin, moles, any areas that is infected or reddened, birthmarks, genital warts, warts with hair growing from them, warts on the face, warts on mucous membranes (such as inside the mouth, nose, anus, genitals, or lips)
Ask A Doctor
Stop and ask a Doctor if discomfort persists. If swallowed get medical help or contact a poison control center right away. Ask a doctor before use if you have diabetes or poor blood circulation.
Package Label

Verucide Physician FormulaSalicyllic Acid LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||