VIDEX EC
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VIDEX EC safely and effectively. See full prescribing information for VIDEX EC. VIDEX EC (didanosine, USP) Delayed-Release Capsules Enteric-Coated Beadlets Initial U.S. Approval: 1991BOXED WARNING WARNING: PANCREATITIS, LACTIC ACIDOSIS andHEPATOMEGALY with STEATOSIS See full prescribing information for complete boxed warning. Fatal and nonfatal pancreatitis. VIDEX EC should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis. (5.1) Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine. (5.2) INDICATIONS AND USAGEVIDEX EC (didanosine, USP) is a nucleoside reverse transcriptase inhibitor for use in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV)-1 infection. (1) DOSAGE AND ADMINISTRATION Adult patients: Administered on an empty stomach. Dosing is based on body weight. (2.1) Pediatric patients: Ages 6 to 18 years, can safely swallow capsules and body weight at least 20 kg. Administered on an empty stomach, dosing is based on body weight. (2.1) Body Weight Dose 20 kg to less than 25 kg 200 mg once daily 25 kg to less than 60 kg 250 mg once daily at least 60 kg 400 mg once daily Renal impairment: Dose reduction is recommended. (2.2) Coadministration with tenofovir: Dose reduction is recommended. Patients should be monitored closely for didanosine-associated adverse reactions. (2.3, 7.1) DOSAGE FORMS AND STRENGTHSCapsules: 125 mg, 200 mg, 250 mg, 400 mg (3) CONTRAINDICATIONSCoadministration with allopurinol or ribavirin is contraindicated. (4.1 and 4.2) WARNINGS AND PRECAUTIONS Pancreatitis: Suspension or discontinuation of didanosine may be necessary. (5.1) Lactic acidosis and severe hepatomegaly with steatosis: Suspend didanosine in patients who develop clinical symptoms or signs with or without laboratory findings. (5.2) Hepatic toxicity: Interruption or discontinuation of didanosine must be considered upon worsening of liver disease. (5.3) Non-cirrhotic portal hypertension: Discontinue didanosine in patients with evidence of non-cirrhotic portal hypertension. (5.4) Patients may develop peripheral neuropathy (5.5), retinal changes and optic neuritis (5.6), immune reconstitution syndrome (5.7), and redistribution/accumulation of body fat (5.8). Side Effects In adults, the most common adverse reactions (greater than 10%, all grades) are diarrhea, peripheral neurologic symptoms/neuropathy, nausea, headache, rash, and vomiting. (6.1) Adverse reactions in pediatric patients were consistent with those in adults. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONSCoadministration of VIDEX EC can alter the concentration of other drugs and other drugs may alter the concentration of didanosine. The potential drug-drug interactions must be considered prior to and during therapy. (4, 7, 12.3) USE IN SPECIFIC POPULATIONS Pregnancy: Fatal lactic acidosis has been reported in pregnant women who received both didanosine and stavudine with other agents. This combination should be used with caution during pregnancy and only if the potential benefit clearly outweighs the potential risk. (5.2, 8.1) Physicians are encouraged to register patients in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: PANCREATITIS, LACTIC ACIDOSIS AND HEPATOMEGALY WITH STEATOSIS
- 1 VIDEX EC INDICATIONS AND USAGE
- 2 VIDEX EC DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 VIDEX EC CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 VIDEX EC ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 VIDEX EC DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
WARNING: PANCREATITIS, LACTIC ACIDOSIS AND HEPATOMEGALY WITH STEATOSIS
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. VIDEX EC should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis [see Warnings and Precautions (5.1) ].
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk [see Warnings and Precautions (5.2) ].
1 INDICATIONS AND USAGE
VIDEX® EC (didanosine, USP), also known as ddI, in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus (HIV)-1 infection [see Clinical Studies (14) ].
2 DOSAGE AND ADMINISTRATION
VIDEX EC should be administered on an empty stomach. VIDEX EC Delayed-Release Capsules should be swallowed intact.
2.1 Recommended Dosage (Adult and Pediatric Patients)
The recommended total daily dose is based on body weight and is administered as one capsule given on a once-daily schedule as outlined in Table 1.
The recommended total daily dose to be administered once daily to pediatric patients weighing at least 20 kg who can swallow capsules is based on body weight (kg), consistent with the recommended adult dosing guidelines (see Table 1). Please consult the complete prescribing information for VIDEX (didanosine) Pediatric Powder for Oral Solution for dosage and administration of didanosine to pediatric patients weighing less than 20 kg or who can not swallow capsules.
| Body Weight | Dose |
| 20 kg to less than 25 kg | 200 mg once daily |
| 25 kg to less than 60 kg | 250 mg once daily |
| at least 60 kg | 400 mg once daily |
2.2 Renal Impairment
Dosing recommendations for VIDEX EC and VIDEX Pediatric Powder for Oral Solution are different for patients with renal impairment. Please consult the complete prescribing information on administration of VIDEX (didanosine) Pediatric Powder for Oral Solution to patients with renal impairment.
Adult Patients
In adult patients with impaired renal function, the dose of VIDEX EC should be adjusted to compensate for the slower rate of elimination. The recommended doses and dosing intervals of VIDEX EC in adult patients with renal insufficiency are presented in Table 2.
|
Creatinine
Clearance (mL/min) |
Dosage (mg) | |
|---|---|---|
| at least 60 kg | less than 60 kg | |
| a Based on studies using a buffered formulation of didanosine. | ||
| b Not suitable for use in patients less than 60 kg with CLcr less than 10 mL/min. An alternate formulation of didanosine should be used. | ||
| at least 60 | 400 once daily | 250 once daily |
| 30-59 | 200 once daily | 125 once daily |
| 10-29 | 125 once daily | 125 once daily |
| less than 10 | 125 once daily | b |
Pediatric Patients
Urinary excretion is also a major route of elimination of didanosine in pediatric patients, therefore the clearance of didanosine may be altered in pediatric patients with renal impairment. Although there are insufficient data to recommend a specific dose adjustment of VIDEX EC in this patient population, a reduction in the dose should be considered (see Table 2).
Patients Requiring Continuous Ambulatory Peritoneal Dialysis (CAPD) or Hemodialysis
For patients requiring CAPD or hemodialysis, follow dosing recommendations for patients with creatinine clearance of less than 10 mL/min, shown in Table 2. It is not necessary to administer a supplemental dose of didanosine following hemodialysis.
2.3 Dose Adjustment
Concomitant Therapy with Tenofovir Disoproxil Fumarate
In patients who are also taking tenofovir disoproxil fumarate, a dose reduction of VIDEX EC to 250 mg (adults weighing at least 60 kg with creatinine clearance of at least 60 mL/min) or 200 mg (adults weighing less than 60 kg with creatinine clearance of at least 60 mL/min) once daily taken together with tenofovir disoproxil fumarate and a light meal (400 kcalories or less, 20% fat or less) or in the fasted state is recommended. The appropriate dose of VIDEX EC coadministered with tenofovir disoproxil fumarate in patients with creatinine clearance of less than 60 mL/min has not been established [see Drug Interactions (7) and Clinical Pharmacology (12.3) ].
Hepatic Impairment
No dose adjustment is required in patients with hepatic impairment [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3) ].
3 DOSAGE FORMS AND STRENGTHS
VIDEX EC (didanosine, USP) Delayed-Release Capsules are white, opaque capsules as described below:
- 125 mg capsule imprinted with “BMS 125 mg 6671” in Tan
- 200 mg capsule imprinted with “BMS 200 mg 6672” in Green
- 250 mg capsule imprinted with “BMS 250 mg 6673” in Blue
- 400 mg capsule imprinted with “BMS 400 mg 6674” in Red
4 CONTRAINDICATIONS
These recommendations are based on either drug interaction studies or observed clinical toxicities.
4.1 Allopurinol
Coadministration of didanosine and allopurinol is contraindicated because systemic exposures of didanosine are increased, which may increase didanosine-associated toxicity [see Clinical Pharmacology (12.3) ].
4.2 Ribavirin
Coadministration of didanosine and ribavirin is contraindicated because exposures of the active metabolite of didanosine (dideoxyadenosine 5′-triphosphate) are increased. Fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in patients receiving both didanosine and ribavirin.
5 WARNINGS AND PRECAUTIONS
5.1 Pancreatitis
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. VIDEX EC should be suspended in patients with signs or symptoms of pancreatitis and discontinued in patients with confirmed pancreatitis. Patients treated with VIDEX EC in combination with stavudine may be at increased risk for pancreatitis.
When treatment with life-sustaining drugs known to cause pancreatic toxicity is required, suspension of VIDEX EC (didanosine) therapy is recommended. In patients with risk factors for pancreatitis, VIDEX EC should be used with extreme caution and only if clearly indicated. Patients with advanced HIV-1 infection, especially the elderly, are at increased risk of pancreatitis and should be followed closely. Patients with renal impairment may be at greater risk for pancreatitis if treated without dose adjustment. The frequency of pancreatitis is dose related. [See Adverse Reactions (6) .]
5.2 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk [see Use in Specific Populations (8.1) ]. Particular caution should be exercised when administering VIDEX EC to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with VIDEX EC should be suspended in any patient who develops clinical signs or symptoms with or without laboratory findings consistent with symptomatic hyperlactatemia, lactic acidosis, or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.3 Hepatic Toxicity
The safety and efficacy of VIDEX EC have not been established in HIV-infected patients with significant underlying liver disease. During combination antiretroviral therapy, patients with preexisting liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities, including severe and potentially fatal hepatic adverse events, and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Hepatotoxicity and hepatic failure resulting in death were reported during postmarketing surveillance in HIV-infected patients treated with hydroxyurea and other antiretroviral agents. Fatal hepatic events were reported most often in patients treated with the combination of hydroxyurea, didanosine, and stavudine. This combination should be avoided. [See Adverse Reactions (6) .]
5.4 Non-cirrhotic Portal Hypertension
Postmarketing cases of non-cirrhotic portal hypertension have been reported, including cases leading to liver transplantation or death. Cases of didanosine-associated non-cirrhotic portal hypertension were confirmed by liver biopsy in patients with no evidence of viral hepatitis. Onset of signs and symptoms ranged from months to years after start of didanosine therapy. Common presenting features included elevated liver enzymes, esophageal varices, hematemesis, ascites, and splenomegaly.
Patients receiving VIDEX EC should be monitored for early signs of portal hypertension (eg, thrombocytopenia and splenomegaly) during routine medical visits. Appropriate laboratory testing including liver enzymes, serum bilirubin, albumin, complete blood count, and international normalized ratio (INR) and ultrasonography should be considered. VIDEX EC should be discontinued in patients with evidence of non-cirrhotic portal hypertension.
5.5 Peripheral Neuropathy
Peripheral neuropathy, manifested by numbness, tingling, or pain in the hands or feet, has been reported in patients receiving didanosine therapy. Peripheral neuropathy has occurred more frequently in patients with advanced HIV disease, in patients with a history of neuropathy, or in patients being treated with neurotoxic drug therapy, including stavudine. Discontinuation of VIDEX EC should be considered in patients who develop peripheral neuropathy. [See Adverse Reactions (6) .]
5.6 Retinal Changes and Optic Neuritis
Retinal changes and optic neuritis have been reported in patients taking didanosine. Periodic retinal examinations should be considered for patients receiving VIDEX EC [see Adverse Reactions (6) ].
5.7 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including VIDEX EC. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.8 Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections:
- Pancreatitis [see Boxed Warning, Warnings and Precautions (5.1) ]
- Lactic acidosis/severe hepatomegaly with steatosis [see Boxed Warning, Warnings and Precautions (5.2) ]
- Hepatic toxicity [see Warnings and Precautions (5.3) ]
- Non-cirrhotic portal hypertension [see Warnings and Precautions (5.4) ]
- Peripheral neuropathy [see Warnings and Precautions (5.5) ]
- Retinal changes and optic neuritis [see Warnings and Precautions (5.6) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
Study AI454-152 was a 48-week, randomized, open-label study comparing VIDEX EC (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients. Selected clinical adverse reactions that occurred in combination with other antiretroviral agents are provided in Table 3.
| Adverse Reactions | Percent of Patientsb,c | |
|---|---|---|
|
VIDEX
EC + stavudine + nelfinavir n=258 |
zidovudine/lamivudined
+ nelfinavir n=253 |
|
| a Median duration of treatment was 62 weeks in the VIDEX EC + stavudine + nelfinavir group and 61 weeks in the zidovudine/lamivudine + nelfinavir group. | ||
| b Percentages based on treated patients. | ||
| c The incidences reported included all severity grades and all reactions regardless of causality. | ||
| d Zidovudine/lamivudine combination tablet. | ||
| * This event was not observed in this study arm. | ||
| Diarrhea | 57 | 58 |
| Peripheral Neurologic Symptoms/Neuropathy | 25 | 11 |
| Nausea | 24 | 36 |
| Headache | 22 | 17 |
| Rash | 14 | 12 |
| Vomiting | 14 | 19 |
| Pancreatitis (see below) | less than 1 | * |
In clinical trials using a buffered formulation of didanosine, pancreatitis resulting in death was observed in one patient who received didanosine plus stavudine plus nelfinavir, one patient who received didanosine plus stavudine plus indinavir, and 2 of 68 patients who received didanosine plus stavudine plus indinavir plus hydroxyurea. In an early access program, pancreatitis resulting in death was observed in one patient who received VIDEX EC plus stavudine plus hydroxyurea plus ritonavir plus indinavir plus efavirenz [see Warnings and Precautions (5) ].
The frequency of pancreatitis is dose related. In phase 3 studies with buffered formulations of didanosine, incidence ranged from 1% to 10% with doses higher than are currently recommended and 1% to 7% with recommended dose.
Selected laboratory abnormalities that occurred in a study of VIDEX EC in combination with other antiretroviral agents are shown in Table 4.
| Percent of Patientsb | ||||
|---|---|---|---|---|
|
VIDEX EC + stavudine + nelfinavir n=258 |
zidovudine/lamivudinec
+ nelfinavir n=253 |
|||
| Parameter | Grades 3-4d | All Grades | Grades 3-4d | All Grades |
| a Median duration of treatment was 62 weeks in the VIDEX EC + stavudine + nelfinavir group and 61 weeks in the zidovudine/lamivudine + nelfinavir group. | ||||
| b Percentages based on treated patients. | ||||
| c Zidovudine/lamivudine combination tablet. | ||||
| d Greater than 5 x ULN for SGOT and SGPT, at least 2.1 x ULN for lipase, and at least 2.6 x ULN for bilirubin (ULN = upper limit of normal). | ||||
| SGOT (AST) | 5 | 46 | 5 | 19 |
| SGPT (ALT) | 6 | 44 | 5 | 22 |
| Lipase | 5 | 23 | 2 | 13 |
| Bilirubin | less than 1 | 9 | less than 1 | 3 |
Pediatric Patients
In clinical trials, 743 pediatric patients between 2 weeks and 18 years of age have been treated with didanosine. Adverse reactions and laboratory abnormalities reported to occur in these patients were generally consistent with the safety profile of didanosine in adults.
In pediatric phase 1 studies, pancreatitis occurred in 2 of 60 (3%) patients treated at entry doses below 300 mg/m2/day and in 5 of 38 (13%) patients treated at higher doses. In study ACTG 152, pancreatitis occurred in none of the 281 pediatric patients who received didanosine 120 mg/m2 every 12 hours and in less than 1% of the 274 pediatric patients who received didanosine 90 mg/m2 every 12 hours in combination with zidovudine [see Clinical Studies (14) ].
Retinal changes and optic neuritis have been reported in pediatric patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of didanosine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to didanosine, or a combination of these factors.
-
-
-
-
-
-
-
-
Use with Stavudine- and Hydroxyurea-Based Regimens
When didanosine is used in combination with other agents with similar toxicities, the incidence of these toxicities may be higher than when didanosine is used alone. Thus, patients treated with VIDEX EC in combination with stavudine, with or without hydroxyurea, may be at increased risk for pancreatitis and hepatotoxicity, which may be fatal, and severe peripheral neuropathy [see Warnings and Precautions (5) ]. The combination of VIDEX EC and hydroxyurea, with or without stavudine, should be avoided.
7 DRUG INTERACTIONS
7.1 Established Drug Interactions
Clinical recommendations based on the results of drug interaction studies are listed in Table 5. Pharmacokinetic results of drug interaction studies are shown in Tables 9-12 [see Contraindications (4.1 and 4.2), Clinical Pharmacology (12.3) ].
| Drug | Effect | Clinical Comment |
|---|---|---|
| ↑ Indicates increase. | ||
| ↓ Indicates decrease. | ||
| a Coadministration of didanosine with food decreases didanosine concentrations. Thus, although not studied, it is possible that coadministration with heavier meals could reduce didanosine concentrations further. | ||
| ganciclovir | ↑ didanosine concentration | If there is no suitable alternative to ganciclovir, then use in combination with VIDEX EC with caution. Monitor for didanosine-associated toxicity. |
| methadone | ↓ didanosine concentration | If coadministration of methadone and didanosine is necessary, the recommended formulation of didanosine is VIDEX EC. Patients should be closely monitored for adequate clinical response when VIDEX EC is coadministered with methadone, including monitoring for changes in HIV RNA viral load. Do not coadminister methadone with VIDEX pediatric powder due to significant decreases in didanosine concentrations. |
| nelfinavir | No interaction 1 hour after didanosine | Administer nelfinavir 1 hour after VIDEX EC. |
| tenofovir disoproxil fumarate | ↑ didanosine concentration | A dose reduction of VIDEX EC to the
following dosage once daily taken together with tenofovir disoproxil fumarate
and a light meal (400 kcalories or less and 20% fat or less) or in the fasted
state is recommended.a
|
Exposure to didanosine is increased when coadministered with tenofovir disoproxil fumarate [Table 5 and see Clinical Pharmacokinetics (12.3, Tables 9 and 10) ]. Increased exposure may cause or worsen didanosine-related clinical toxicities, including pancreatitis, symptomatic hyperlactatemia/lactic acidosis, and peripheral neuropathy. Coadministration of tenofovir disoproxil fumarate with VIDEX EC should be undertaken with caution, and patients should be monitored closely for didanosine-related toxicities and clinical response. VIDEX EC should be suspended if signs or symptoms of pancreatitis, symptomatic hyperlactatemia, or lactic acidosis develop [see Dosage and Administration (2.3), Warnings and Precautions (5) ]. Suppression of CD4 cell counts has been observed in patients receiving tenofovir disoproxil fumarate with didanosine at a dose of 400 mg daily.
7.2 Predicted Drug Interactions
Predicted drug interactions with VIDEX EC are listed in Table 6.
| Drug or Drug Class | Effect | Clinical Comment |
|---|---|---|
| ↑ Indicates increase. | ||
| a Only if other drugs are not available and if clearly indicated. If treatment with life-sustaining drugs that cause pancreatic toxicity is required, suspension of VIDEX EC is recommended [see Warnings and Precautions (5.1) ]. | ||
| b [See Warnings and Precautions (5.6) .] | ||
| Drugs that may cause pancreatic toxicity | ↑ risk of pancreatitis | Use only with extreme caution.a |
| Neurotoxic drugs | ↑ risk of neuropathy | Use with caution.b |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies have been performed in rats and rabbits at doses up to 12 and 14.2 times the estimated human exposure (based upon plasma levels), respectively, and have revealed no evidence of impaired fertility or harm to the fetus due to didanosine. At approximately 12 times the estimated human exposure, didanosine was slightly toxic to female rats and their pups during mid and late lactation. These rats showed reduced food intake and body weight gains but the physical and functional development of the offspring was not impaired and there were no major changes in the F2 generation. A study in rats showed that didanosine and/or its metabolites are transferred to the fetus through the placenta. Animal reproduction studies are not always predictive of human response.
There are no adequate and well-controlled studies of didanosine in pregnant women. Didanosine should be used during pregnancy only if the potential benefit justifies the potential risk.
Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. It is unclear if pregnancy augments the risk of lactic acidosis/hepatic steatosis syndrome reported in nonpregnant individuals receiving nucleoside analogues [see Warnings and Precautions (5.2) ]. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk. Healthcare providers caring for HIV-infected pregnant women receiving didanosine should be alert for early diagnosis of lactic acidosis/hepatic steatosis syndrome.
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant women exposed to didanosine and other antiretroviral agents, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
8.3 Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. A study in rats showed that following oral administration, didanosine and/or its metabolites were excreted into the milk of lactating rats. It is not known if didanosine is excreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving didanosine.
8.4 Pediatric Use
Use of didanosine in pediatric patients from 2 weeks of age through adolescence is supported by evidence from adequate and well-controlled studies of didanosine in adult and pediatric patients [see Dosage and Administration (2), Adverse Reactions (6.1), Clinical Pharmacology (12.3) , and Clinical Studies (14) ]. Additional pharmacokinetic studies in pediatric patients support use of VIDEX EC in pediatric patients who weigh at least 20 kg.
8.5 Geriatric Use
In an Expanded Access Program using a buffered formulation of didanosine for the treatment of advanced HIV infection, patients aged 65 years and older had a higher frequency of pancreatitis (10%) than younger patients (5%) [see Warnings and Precautions (5.1) ]. Clinical studies of didanosine, including those for VIDEX EC, did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently than younger subjects. Didanosine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly [see Dosage and Administration (2.2) ].
8.6 Renal Impairment
Patients with renal impairment (creatinine clearance of less than 60 mL/min) may be at greater risk of toxicity from didanosine due to decreased drug clearance [see Clinical Pharmacology (12.3) ]. A dose reduction is recommended for these patients [see Dosage and Administration (2) ].
10 OVERDOSAGE
There is no known antidote for didanosine overdosage. In phase 1 studies, in which buffered formulations of didanosine were initially administered at doses ten times the currently recommended dose, toxicities included: pancreatitis, peripheral neuropathy, diarrhea, hyperuricemia, and hepatic dysfunction. Didanosine is not dialyzable by peritoneal dialysis, although there is some clearance by hemodialysis [see Clinical Pharmacology (12.3) ].
11 DESCRIPTION
VIDEX® EC is the brand name for an enteric-coated formulation of didanosine, USP, a synthetic purine nucleoside analogue active against HIV-1. VIDEX EC Delayed-Release Capsules, containing enteric-coated beadlets, are available for oral administration in strengths of 125, 200, 250, and 400 mg of didanosine. The inactive ingredients in the beadlets include carboxymethylcellulose sodium 12, diethyl phthalate, methacrylic acid copolymer, sodium hydroxide, sodium starch glycolate, and talc. The capsule shells contain gelatin and titanium dioxide. The capsules are imprinted with edible inks.
Didanosine is also available in a powder formulation. Please consult the prescribing information for VIDEX (didanosine) Pediatric Powder for Oral Solution for additional information.
The chemical name for didanosine is 2′,3′-dideoxyinosine. The structural formula is:
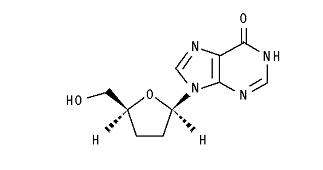
Didanosine is a white crystalline powder with the molecular formula C10H12N4O3 and a molecular weight of 236.2. The aqueous solubility of didanosine at 25° C and pH of approximately 6 is 27.3 mg/mL. Didanosine is unstable in acidic solutions. For example, at pH less than 3 and 37° C, 10% of didanosine decomposes to hypoxanthine in less than 2 minutes. In VIDEX EC, an enteric coating is used to protect didanosine from degradation by stomach acid.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Didanosine is an antiviral agent [see Clinical Pharmacology (12.4) ].
12.3 Pharmacokinetics
The pharmacokinetic parameters of didanosine in HIV-infected adult and pediatric patients are summarized in Table 7, by weight ranges that correspond to recommended doses (Table 1). Didanosine is rapidly absorbed, with peak plasma concentrations generally observed from 0.25 to 1.50 hours following oral dosing with a buffered formulation. Increases in plasma didanosine concentrations were dose proportional over the range of 50 to 400 mg. In adults, the mean (± standard deviation) oral bioavailability following single oral dosing with a buffered formulation is 42 (±12)%. After oral administration, the urinary recovery of didanosine is approximately 18 (±8)% of the dose. The CSF-plasma ratio following IV administration is 21 (±0.03)%. Steady-state pharmacokinetic parameters did not differ significantly from values obtained after a single dose. Binding of didanosine to plasma proteins in vitro was low (less than 5%). Based on data from in vitro and animal studies, it is presumed that the metabolism of didanosine in man occurs by the same pathways responsible for the elimination of endogenous purines.
| a The pharmacokinetic parameters (mean ± standard deviation) of didanosine were determined by a population pharmacokinetic model based on combined clinical studies. | ||||
| Parametera | Pediatrics | Adults | ||
|---|---|---|---|---|
| 20 kg to less than 25 kg n=10 |
25 kg to less than 60 kg n=17 |
At least 60 kg
n=7 |
At least 60 kg
n=44 |
|
| Apparent clearance (L/h) | 89.5 ± 21.6 | 116.2 ± 38.6 | 196.0 ± 55.8 | 174.5 ± 69.7 |
| Apparent volume of distribution (L) | 98.1 ± 30.2 | 154.7 ± 55.0 | 363 ± 137.7 | 308.3 ± 164.3 |
| Elimination half-life (h) | 0.75 ± 0.13 | 0.92 ± 0.09 | 1.26 ± 0.19 | 1.19 ± 0.21 |
| Steady-state AUC (mg•h/L) | 2.38 ± 0.66 | 2.36 ± 0.70 | 2.25 ± 0.89 | 2.65 ± 1.07 |
Comparison of Didanosine Formulations
In VIDEX EC, the active ingredient, didanosine, is protected against degradation by stomach acid by the use of an enteric coating on the beadlets in the capsule. The enteric coating dissolves when the beadlets empty into the small intestine, the site of drug absorption. With buffered formulations of didanosine, administration with antacid provides protection from degradation by stomach acid.
In healthy volunteers, as well as subjects infected with HIV-1, the AUC is equivalent for didanosine administered as the VIDEX EC formulation relative to a buffered tablet formulation. The peak plasma concentration (Cmax) of didanosine, administered as VIDEX EC, is reduced approximately 40% relative to didanosine buffered tablets. The time to the peak concentration (Tmax) increases from approximately 0.67 hours for didanosine buffered tablets to 2.0 hours for VIDEX EC.
Effect of Food
In the presence of food, the Cmax and AUC for VIDEX EC were reduced by approximately 46% and 19%, respectively, compared to the fasting state [see Dosage and Administration (2) ]. VIDEX EC should be taken on an empty stomach.
Special Populations
Renal Insufficiency: Data from two studies using a buffered formulation of didanosine indicated that the apparent oral clearance of didanosine decreased and the terminal elimination half-life increased as creatinine clearance decreased (see Table 8). Following oral administration, didanosine was not detectable in peritoneal dialysate fluid (n=6); recovery in hemodialysate (n=5) ranged from 0.6% to 7.4% of the dose over a 3-4 hour dialysis period. The absolute bioavailability of didanosine was not affected in patients requiring dialysis. [See Dosage and Administration (2.2) .]
| Creatinine Clearance (mL/min) | |||||
|---|---|---|---|---|---|
| Parameter |
at least
90 n=12 |
60-90 n=6 |
30-59 n=6 |
10-29 n=3 |
Dialysis
Patients n=11 |
| ND = not determined due to anuria. | |||||
| CLcr = creatinine clearance. | |||||
| CL/F = apparent oral clearance. | |||||
| CLR = renal clearance. | |||||
| CLcr (mL/min) | 112 ± 22 | 68 ± 8 | 46 ± 8 | 13 ± 5 | ND |
| CL/F (mL/min) | 2164 ± 638 | 1566 ± 833 | 1023 ± 378 | 628 ± 104 | 543 ± 174 |
| CLR (mL/min) | 458 ± 164 | 247 ± 153 | 100 ± 44 | 20 ± 8 | less than 10 |
| T½ (h) | 1.42 ± 0.33 | 1.59 ± 0.13 | 1.75 ± 0.43 | 2.0 ± 0.3 | 4.1 ± 1.2 |
Hepatic Impairment: The pharmacokinetics of didanosine have been studied in 12 non-HIV-infected subjects with moderate (n=8) to severe (n=4) hepatic impairment (Child-Pugh Class B or C). Mean AUC and Cmax values following a single 400 mg dose of didanosine were approximately 13% and 19% higher, respectively, in patients with hepatic impairment compared to matched healthy subjects. No dose adjustment is needed, because a similar range and distribution of AUC and Cmax values was observed for subjects with hepatic impairment and matched healthy controls. [See Dosage and Administration (2.3) .]
Pediatric Patients: The pharmacokinetics of didanosine have been evaluated in HIV-exposed and HIV-infected pediatric patients from birth to adulthood.
A population pharmacokinetic analysis was conducted on pooled didanosine plasma concentration data from 9 clinical trials in 106 pediatric (neonate to 18 years of age) and 45 adult patients (greater than 18 years of age). Results showed that body weight is the primary factor associated with oral clearance. Based on the data analyzed, dosing schedule (once versus twice daily) and formulation (powder for oral solution, tablet, and delayed-release capsule) did not have an effect on oral clearance. Didanosine exposure similar to that at recommended adult doses can be achieved in pediatric patients with a weight-based dosing scheme [see Dosage and Administration (2) ].
Geriatric Patients: Didanosine pharmacokinetics have not been studied in patients over 65 years of age [see Use in Specific Populations (8.5) ].
Gender: The effects of gender on didanosine pharmacokinetics have not been studied.
Drug Interactions
Tables 9 and 10 summarize the effects on AUC and Cmax, with a 90% confidence interval (CI) when available, following coadministration of VIDEX EC with a variety of drugs. For clinical recommendations based on drug interaction studies for drugs in bold font, see Dosage and Administration (2.3) and Drug Interactions (7.1) .
| % Change of Didanosine Pharmacokinetic Parametersa |
||||
|---|---|---|---|---|
| Drug | Didanosine Dosage | n |
AUC of Didanosine (90% CI) |
Cmax of Didanosine (90% CI) |
| ↑ Indicates increase. | ||||
| ↓ Indicates decrease. | ||||
| ↔ Indicates no change, or mean increase or decrease of less than 10%. | ||||
| a The 90% confidence intervals for the percent change in the pharmacokinetic parameter are displayed. | ||||
| b All studies conducted in healthy volunteers at least 60 kg with creatinine clearance of at least 60 mL/min. | ||||
| c Tenofovir disoproxil fumarate. | ||||
| d 373 kcalories, 8.2 grams fat. | ||||
| e Compared with VIDEX EC 250 mg administered alone under fasting conditions. | ||||
| f Compared with VIDEX EC 400 mg administered alone under fasting conditions. | ||||
| g Comparisons are made to historical controls (n=148, pooled from 5 studies) conducted in healthy subjects. The number of subjects evaluated for AUC and Cmax is 15 and 16, respectively. | ||||
|
tenofovir,b,c 300 mg once daily with a light meald |
400 mg single dose fasting 2 hours before tenofovir |
26 | ↑ 48% (31, 67%) |
↑ 48% (25, 76%) |
|
tenofovir,b,c 300 mg once daily with a light meald |
400 mg single dose with tenofovir and a light meal |
25 | ↑ 60% (44, 79%) |
↑ 64% (41, 89%) |
|
tenofovir,b,c 300 mg once daily with a light meald |
200 mg single dose with tenofovir and a light meal |
33 | ↑ 16% (6, 27%)e |
↓ 12% (-25, 3%)e |
| 250 mg single dose with tenofovir and a light meal |
33 | ↔ (-13, 5%)f |
↓ 20% (-32, -7%)f |
|
| 325 mg single dose with tenofovir and a light meal |
33 | ↑ 13% (3, 24%)f |
↓ 11% (-24, 4%)f |
|
| methadone, chronic maintenance dose | 400 mg single dose | 15, 16g | ↓ 17% (-29, -2%) |
↓ 16% (-33, 4%) |
| % Change of Coadministered Drug Pharmacokinetic Parametersa,b |
||||
|---|---|---|---|---|
| Drug | Didanosine Dosage | n |
AUC of Coadministered Drug (90% CI) |
Cmax of Coadministered Drug (90% CI) |
| ↔ Indicates no change, or mean increase or decrease of less than 10%. | ||||
| a The 90% confidence intervals for the percent change in the pharmacokinetic parameter are displayed. | ||||
| b All studies conducted in healthy volunteers at least 60 kg with creatinine clearance of at least 60 mL/min. | ||||
| c Tenofovir disoproxil fumarate. | ||||
| d 373 kcalories, 8.2 grams fat. | ||||
| ciprofloxacin, 750 mg single dose |
400 mg single dose | 16 | ↔ | ↔ |
| indinavir, 800 mg single dose |
400 mg single dose | 23 | ↔ | ↔ |
| ketoconazole, 200 mg single dose |
400 mg single dose | 21 | ↔ | ↔ |
| tenofovir,c 300 mg once daily with a light meald |
400 mg single dose fasting 2 hours before tenofovir |
25 | ↔ | ↔ |
| tenofovir,c 300
mg once daily with a light meald |
400 mg single dose with tenofovir and a light meal |
25 | ↔ | ↔ |
Didanosine Buffered Formulations: Tables 11 and 12 summarize the effects on AUC and Cmax, with a 90% or 95% CI when available, following coadministration of buffered formulations of didanosine with a variety of drugs. The results of these studies may be expected to apply to VIDEX EC. For most of the listed drugs, no clinically significant pharmacokinetic interactions were noted. For clinical recommendations based on drug interaction studies for drugs in bold font, see Dosage and Administration (2.3 for Concomitant Therapy with Tenofovir Disoproxil Fumarate), Contraindications (4.1), and Drug Interactions (7.1) .
| % Change of Didanosine Pharmacokinetic Parametersa |
||||
|---|---|---|---|---|
| Drug | Didanosine Dosage | n |
AUC of Didanosine (95% CI) |
Cmax of Didanosine (95% CI) |
| ↑ Indicates increase. | ||||
| ↓ Indicates decrease. | ||||
| ↔ Indicates no change, or mean increase or decrease of less than 10%. | ||||
| a The 95% confidence intervals for the percent change in the pharmacokinetic parameter are displayed. | ||||
| b 90% CI. | ||||
| c HIV-infected patients. | ||||
| NA = Not available. | ||||
|
allopurinol, renally impaired, 300 mg/day |
200 mg single dose | 2 | ↑ 312% | ↑ 232% |
| healthy volunteer, 300 mg/day for 7 days |
400 mg single dose | 14 | ↑ 113% | ↑ 69% |
|
ganciclovir, 1000 mg
every 8 hours, 2 hours after didanosine |
200 mg every 12 hours | 12 | ↑ 111% | NA |
| ciprofloxacin, 750 mg every 12 hours for 3 days, 2 hours before didanosine | 200 mg every 12 hours for 3 days |
8c | ↓ 16% | ↓ 28% |
| indinavir, 800 mg single dose | ||||
| simultaneous | 200 mg single dose | 16 | ↔ | ↔ |
| 1 hour before didanosine | 200 mg single dose | 16 | ↓ 17% (-27, -7%)b |
↓ 13% (-28, 5%)b |
| ketoconazole, 200 mg/day for 4 days, 2 hours before didanosine |
375 mg every 12 hours for 4 days |
12c | ↔ | ↓ 12% |
| loperamide, 4 mg every 6 hours for 1 day | 300 mg single dose | 12c | ↔ | ↓ 23% |
| metoclopramide, 10 mg single dose | 300 mg single dose | 12c | ↔ | ↑ 13% |
| ranitidine, 150 mg single dose, 2 hours before didanosine |
375 mg single dose | 12c | ↑ 14% | ↑ 13% |
| rifabutin, 300 mg or 600 mg/day for 12 days | 167 mg or 250 mg every 12 hours for 12 days |
11 | ↑ 13% (-1, 27%) |
↑ 17% (-4, 38%) |
| ritonavir, 600 mg every 12 hours for 4 days | 200 mg every 12 hours for 4 days |
12 | ↓ 13% (0, 23%) |
↓ 16% (5, 26%) |
| stavudine, 40 mg every 12 hours for 4 days | 100 mg every 12 hours for 4 days |
10 | ↔ | ↔ |
| sulfamethoxazole, 1000 mg single dose | 200 mg single dose | 8c | ↔ | ↔ |
| trimethoprim, 200 mg single dose | 200 mg single dose | 8c | ↔ | ↑ 17% (-23, 77%) |
| zidovudine, 200 mg every 8 hours for 3 days | 200 mg every 12 hours for 3 days |
6c | ↔ | ↔ |
| % Change of Coadministered Drug Pharmacokinetic Parametersa |
||||
|---|---|---|---|---|
| Drug | Didanosine Dosage | n |
AUC
of Coadministered Drug (95% CI) |
Cmax of
Coadministered Drug (95% CI) |
| ↑ Indicates increase. | ||||
| ↓ Indicates decrease. | ||||
| ↔ Indicates no change, or mean increase or decrease of less than 10%. | ||||
| a The 95% confidence intervals for the percent change in the pharmacokinetic parameter are displayed. | ||||
| b HIV-infected patients. | ||||
| NA = Not available. | ||||
| dapsone, 100 mg single dose | 200 mg every 12 hours for 14 days | 6b | ↔ | ↔ |
| ganciclovir, 1000 mg every 8 hours, 2 hours after didanosine |
200 mg every 12 hours | 12b | ↓ 21% | NA |
|
nelfinavir, 750 mg single dose, 1 hour after didanosine |
200 mg single dose | 10b | ↑ 12% | ↔ |
| ranitidine, 150 mg single dose, 2 hours before didanosine |
375 mg single dose | 12b | ↓ 16% | ↔ |
| ritonavir, 600 mg every 12 hours for 4 days | 200 mg every 12 hours for 4 days |
12 | ↔ | ↔ |
| stavudine, 40 mg every 12 hours for 4 days | 100 mg every 12 hours for 4 days |
10b | ↔ | ↑ 17% |
| sulfamethoxazole, 1000 mg single dose | 200 mg single dose | 8b | ↓ 11% (-17, -4%) |
↓ 12% (-28, 8%) |
| trimethoprim, 200 mg single dose | 200 mg single dose | 8b | ↑ 10% (-9, 34%) |
↓ 22% (-59, 49%) |
| zidovudine, 200 mg every 8 hours for 3 days | 200 mg every 12 hours for 3 days |
6b | ↓ 10% (-27, 11%) |
↓ 16.5% (-53, 47%) |
12.4 Microbiology
Mechanism of Action
Didanosine is a synthetic nucleoside analogue of the naturally occurring nucleoside deoxyadenosine in which the 3′-hydroxyl group is replaced by hydrogen. Intracellularly, didanosine is converted by cellular enzymes to the active metabolite, dideoxyadenosine 5′-triphosphate. Dideoxyadenosine 5′-triphosphate inhibits the activity of HIV-1 reverse transcriptase both by competing with the natural substrate, deoxyadenosine 5′-triphosphate, and by its incorporation into viral DNA causing termination of viral DNA chain elongation.
Antiviral Activity in Cell Culture
The anti-HIV-1 activity of didanosine was evaluated in a variety of HIV-1 infected lymphoblastic cell lines and monocyte/macrophage cell cultures. The concentration of drug necessary to inhibit viral replication by 50% (EC50) ranged from 2.5 to 10 μM (1 μM = 0.24 μg/mL) in lymphoblastic cell lines and 0.01 to 0.1 μM in monocyte/macrophage cell cultures.
Resistance
HIV-1 isolates with reduced sensitivity to didanosine have been selected in cell culture and were also obtained from patients treated with didanosine. Genetic analysis of isolates from didanosine-treated patients showed mutations in the reverse transcriptase gene that resulted in the amino acid substitutions K65R, L74V, and M184V. The L74V substitution was most frequently observed in clinical isolates. Phenotypic analysis of HIV-1 isolates from 60 patients (some with prior zidovudine treatment) receiving 6 to 24 months of didanosine monotherapy showed that isolates from 10 of 60 patients exhibited an average of a 10-fold decrease in susceptibility to didanosine in cell culture compared to baseline isolates. Clinical isolates that exhibited a decrease in didanosine susceptibility harbored one or more didanosine resistance-associated substitutions.
Cross-resistance
HIV-1 isolates from 2 of 39 patients receiving combination therapy for up to 2 years with didanosine and zidovudine exhibited decreased susceptibility to didanosine, lamivudine, stavudine, zalcitabine, and zidovudine in cell culture. These isolates harbored five substitutions (A62V, V75I, F77L, F116Y, and Q151M) in the reverse transcriptase gene. In data from clinical studies, the presence of thymidine analogue mutations (M41L, D67N, L210W, T215Y, K219Q) has been shown to decrease the response to didanosine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were conducted in mice and rats for 22 and 24 months, respectively. In the mouse study, initial doses of 120, 800, and 1200 mg/kg/day for each sex were lowered after 8 months to 120, 210, and 210 mg/kg/day for females and 120, 300, and 600 mg/kg/day for males. The two higher doses exceeded the maximally tolerated dose in females and the high dose exceeded the maximally tolerated dose in males. The low dose in females represented 0.68-fold maximum human exposure and the intermediate dose in males represented 1.7-fold maximum human exposure based on relative AUC comparisons. In the rat study, initial doses were 100, 250, and 1000 mg/kg/day, and the high dose was lowered to 500 mg/kg/day after 18 months. The upper dose in male and female rats represented 3-fold maximum human exposure.
Didanosine induced no significant increase in neoplastic lesions in mice or rats at maximally tolerated doses.
Didanosine was positive in the following genetic toxicology assays: 1) the Escherichia coli tester strain WP2 uvrA bacterial mutagenicity assay; 2) the L5178Y/TK+/- mouse lymphoma mammalian cell gene mutation assay; 3) the in vitro chromosomal aberrations assay in cultured human peripheral lymphocytes; 4) the in vitro chromosomal aberrations assay in Chinese Hamster Lung cells; and 5) the BALB/c 3T3 in vitro transformation assay. No evidence of mutagenicity was observed in an Ames Salmonella bacterial mutagenicity assay or in rat and mouse in vivo micronucleus assays.
13.2 Animal Toxicology and/or Pharmacology
Evidence of a dose-limiting skeletal muscle toxicity has been observed in mice and rats (but not in dogs) following long-term (greater than 90 days) dosing with didanosine at doses that were approximately 1.2 to 12 times the estimated human exposure. The relationship of this finding to the potential of didanosine to cause myopathy in humans is unclear. However, human myopathy has been associated with administration of didanosine and other nucleoside analogues.
14 CLINICAL STUDIES
14.1 Adult Patients
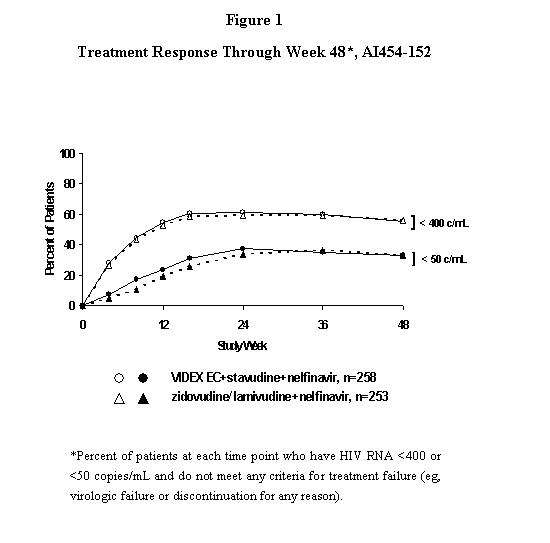
Study Al454-152 was a 48-week, randomized, open-label study comparing VIDEX EC (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients, with a mean CD4 cell count of 411 cells/mm3 (range 39 to 1105 cells/mm3) and a mean plasma HIV-1 RNA of 4.71 log10 copies/mL (range 2.8 to 5.9 log10 copies/mL) at baseline. Patients were primarily males (72%) and Caucasian (53%) with a mean age of 35 years (range 18 to 73 years). The percentages of patients with HIV-1 RNA less than 400 and less than 50 copies/mL and outcomes of patients through 48 weeks are summarized in Figure 1 and Table 13, respectively.
| Outcome |
Percent of
Patients with HIV-1 RNA less than 400 copies/mL (less than 50 copies/mL) |
|
|---|---|---|
|
VIDEX EC + stavudine + nelfinavir n=258 |
zidovudine/lamivudinea
+ nelfinavir n=253 |
|
| a Zidovudine/lamivudine combination tablet. | ||
| b Corresponds to rates at Week 48 in Figure 1. | ||
| c Subjects achieved and maintained confirmed HIV-1 RNA less than 400 copies/mL (less than 50 copies/mL) through Week 48. | ||
| d Includes viral rebound at or before Week 48 and failure to achieve confirmed HIV-1 RNA less than 400 copies/mL (less than 50 copies/mL) through Week 48. | ||
| e Includes lost to follow-up, subject’s withdrawal, discontinuation due to physician’s decision, never treated, and other reasons. | ||
| Responderb,c | 55% (33%) | 56% (33%) |
| Virologic failured | 22% (45%) | 21% (43%) |
| Death or discontinued due to disease progression | 1% (1%) | 2% (2%) |
| Discontinued due to adverse event | 6% (6%) | 7% (7%) |
| Discontinued due to other reasonse | 16% (16%) | 15% (16%) |
14.2 Pediatric Patients
Efficacy in pediatric patients was demonstrated in a randomized, double-blind, controlled study (ACTG 152, conducted 1991-1995) involving 831 patients 3 months to 18 years of age treated for more than 1.5 years with zidovudine (180 mg/m2 every 6 hours), didanosine (120 mg/m2 every 12 hours), or zidovudine (120 mg/m2 every 6 hours) plus didanosine (90 mg/m2 every 12 hours). Patients treated with didanosine or didanosine plus zidovudine had lower rates of HIV-1 disease progression or death compared with those treated with zidovudine alone.
16 HOW SUPPLIED/STORAGE AND HANDLING
VIDEX EC (didanosine, USP) Delayed-Release Capsules are white, opaque capsules that are packaged in bottles with child-resistant closures as described in Table 14.
| 125 mg capsule imprinted with “BMS 125 mg 6671” in Tan | ||
| NDC No. 0087-6671-17 | 30 capsules/bottle | |
| 200 mg capsule imprinted with “BMS 200 mg 6672” in Green | ||
| NDC No. 0087-6672-17 | 30 capsules/bottle | |
| 250 mg capsule imprinted with “BMS 250 mg 6673” in Blue | ||
| NDC No. 0087-6673-17 | 30 capsules/bottle | |
| 400 mg capsule imprinted with “BMS 400 mg 6674” in Red | ||
| NDC No. 0087-6674-17 | 30 capsules/bottle | |
Storage
The capsules should be stored in tightly closed containers at 25° C (77° F). Excursions between 15° C and 30° C (59° F and 86° F) are permitted (see USP Controlled Room Temperature).
17 PATIENT COUNSELING INFORMATION
See Medication Guide.
17.1 Pancreatitis
Patients should be informed that a serious toxicity of didanosine, used alone and in combination regimens, is pancreatitis, which may be fatal.
17.2 Peripheral Neuropathy
Patients should be informed that peripheral neuropathy, manifested by numbness, tingling, or pain in hands or feet, may develop during therapy with VIDEX EC (didanosine). Patients should be counseled that peripheral neuropathy occurs with greatest frequency in patients with advanced HIV-1 disease or a history of peripheral neuropathy, and that discontinuation of VIDEX EC may be required if toxicity develops.
17.3 Lactic Acidosis and Severe Hepatomegaly with Steatosis
Patients should be informed that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals.
17.4 Hepatic Toxicity
Patients should be informed that hepatotoxicity including fatal hepatic adverse events were reported in patients with preexisting liver dysfunction. The safety and efficacy of VIDEX EC have not been established in HIV-infected patients with significant underlying liver disease.
17.5 Non-cirrhotic Portal Hypertension
Patients should be informed that non-cirrhotic portal hypertension has been reported in patients taking VIDEX EC, including cases leading to liver transplantation or death.
17.6 Retinal Changes and Optic Neuritis
Patients should be informed that retinal changes and optic neuritis have been reported in adult and pediatric patients.
17.7 Fat Redistribution
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
17.8 Concomitant Therapy
Patients should be informed that when didanosine is used in combination with other agents with similar toxicities, the incidence of adverse events may be higher than when didanosine is used alone. These patients should be followed closely.
Patients should be cautioned about the use of medications or other substances, including alcohol, which may exacerbate VIDEX EC toxicities.
17.9 General Information
VIDEX EC is not a cure for HIV-1 infection, and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Therefore, patients should remain under the care of a physician when using VIDEX EC.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. It is not known if VIDEX EC can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in breast milk.
Patients should be instructed to swallow the capsule as a whole and to not open the capsule.
Patients should be instructed to not miss a dose but if they do, patients should take VIDEX EC as soon as possible. Patients should be told that if it is almost time for the next dose, they should skip the missed dose and continue with the regular dosing schedule.
Patients should be instructed to contact a poison control center or emergency room right away in case of an overdose.
Medication Guide
VIDEX
®
EC (VY-dex Ee-see)
(didanosine, also known as ddI)
Delayed-Release Capsules
Enteric-Coated Beadlets
Read this Medication Guide before you start taking VIDEX EC and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. You and your healthcare provider should talk about your treatment with VIDEX EC before you start taking it and at regular check-ups. You should stay under your healthcare provider’s care when taking VIDEX EC.
What is the most important information I should know about VIDEX EC?
VIDEX EC may cause serious side effects, including:
1. Swelling of your pancreas (pancreatitis) that may cause death. Pancreatitis can happen at any time during your treatment with VIDEX EC. Before you start taking VIDEX EC, tell your healthcare provider if you:
- have had pancreatitis
- have advanced HIV (human immunodeficiency virus) infection
- have kidney problems
- drink alcoholic beverages
- take a medicine called ZERIT® (stavudine)
It is important to call your healthcare provider right away if you have:
- stomach pain
- swelling of your stomach
- nausea and vomiting
- fever
2. Build-up of acid in your blood (lactic acidosis). Lactic acidosis must be treated in the hospital as it may cause death. Before you start taking VIDEX EC, tell your healthcare provider if you:
- have liver problems
- are pregnant. There have been deaths reported in pregnant women who get lactic acidosis after taking VIDEX EC and ZERIT (stavudine).
- are overweight
- have been treated for a long time with other medicines to treat HIV
It is important to call your healthcare provider right away if you:
- feel weak or tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have stomach pain with nausea and vomiting
- feel cold, especially in your arms and legs
- feel dizzy or light-headed
- have a fast or irregular heartbeat
3. Liver problems. Serious liver problems have happened in some people (including pregnant women) who take VIDEX EC. These problems include liver enlargement (hepatomegaly), fat in the liver (steatosis), liver failure, and high blood pressure in the large vein of the liver (portal hypertension). Severe liver problems can lead to liver transplantation or death in some people taking VIDEX EC. Your healthcare provider should check your liver function while you are taking VIDEX EC. You should be especially careful if you have a history of heavy alcohol use or liver problems.
It is important to call your healthcare provider right away if you have:
- yellowing of your skin or the white of your eyes (jaundice)
- dark urine
- pain on the right side of your stomach
- swelling of your stomach
- easy bruising or bleeding
- loss of appetite
- nausea or vomiting
- vomiting blood or dark colored stools (bowel movements)
What is VIDEX EC?
VIDEX EC is a prescription medicine used with other antiretroviral medicines to treat human immunodeficiency virus (HIV) infection in children and adults. VIDEX EC belongs to a class of drugs called nucleoside analogues.
VIDEX EC will not cure your HIV infection. At present there is no cure for HIV infection. Even while taking VIDEX EC, you may continue to have HIV-related illnesses, including infections with other disease-producing organisms. Continue to see your healthcare provider regularly and report any medical problems that occur.
Who should not take VIDEX EC?
Do not take VIDEX EC if you take:
- ZYLOPRIM®, LOPURIN®, ALOPRIM® (allopurinol)
- COPEGUS®, REBETOL®, RIBASPHERE®, RIBAVIRIN®, VIRAZOLE® (ribavirin)
What should I tell my healthcare provider before taking VIDEX EC?
Before you take VIDEX EC, tell your healthcare provider if you:
- have or had kidney problems
- have or had liver problems (such as hepatitis)
- have or had persistent numbness, tingling, or pain in the hands or feet (neuropathy)
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if VIDEX EC will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking VIDEX EC. You and your healthcare provider will decide if you should take VIDEX EC while you are pregnant.
Pregnancy Registry: There is a pregnancy registry for women who take antiviral medicines during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. Talk to your doctor about how you can take part in this registry. - are breastfeeding or plan to breastfeed. Do not breastfeed. It is not known if VIDEX EC can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. VIDEX EC may affect the way other medicines work, and other medicines may affect how VIDEX EC works.
Especially tell your healthcare provider if you take:
- CYTOVENE®, VALCYTE® (ganciclovir)
- DOLOPHINE® HYDROCHLORIDE, METHADOSE® (methadone)
- VIRACEPT® (nelfinavir)
- VIREAD® (tenofovir disoproxil fumarate)
- alcoholic beverages
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
Ask your healthcare provider if you are not sure if you take one of the medicines listed above.
How should I take VIDEX EC?
- Take VIDEX EC exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much VIDEX EC to take and when to take it.
- Your healthcare provider may change your dose. Do not change your dose of VIDEX EC without talking to your healthcare provider.
- Do not take VIDEX EC with food. Take VIDEX EC on an empty stomach.
- Take VIDEX EC capsules whole. Do not break, crush, dissolve, or chew VIDEX EC capsules before swallowing. If you cannot swallow VIDEX EC capsules whole, tell your healthcare provider. You may need a different medicine.
- Try not to miss a dose, but if you do, take it as soon as possible. If it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule.
- Some medicines should not be taken at the same time of day that you take VIDEX EC. Check with your healthcare provider.
- If your kidneys are not working well, your healthcare provider will need to do regular blood and urine tests to check how they are working while you take VIDEX EC. Your healthcare provider may also lower your dosage of VIDEX EC if your kidneys are not working well.
- If you take too much VIDEX EC, contact a poison control center or emergency room right away.
What should I avoid while taking VIDEX EC?
- Alcohol. Do not drink alcohol while taking VIDEX EC. Alcohol may increase your risk of getting pain and swelling of your pancreas (pancreatitis) or may damage your liver.
What are the possible side effects of VIDEX EC?
VIDEX EC can cause pancreatitis, lactic acidosis, and liver problems. See “What is the most important information I should know about VIDEX EC?” at the beginning of this Medication Guide.
- Vision changes. You should have regular eye exams while taking VIDEX EC.
- Peripheral neuropathy. Symptoms include: numbness, tingling, or pain in your hands or feet. This condition is more likely to happen in people who have had it before, in patients taking medicines that affect the nerves, and in people with advanced HIV disease. A child may not notice these symptoms. Ask the child’s healthcare provider for the signs and symptoms of peripheral neuropathy in children.
- Changes in your immune system (immune reconstitution syndrome). Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider if you start having new or worse symptoms of infection after you start taking HIV medicine.
-
Changes in body fat (fat redistribution). Changes in body fat have been seen in people who take antiretroviral medicines. These changes may include:
- more fat in or around your
- upper back and neck (buffalo hump)
- breasts or chest
- trunk
- less fat in your
- legs
- arms
- face
- more fat in or around your
Tell your healthcare provider if you have any of the symptoms listed above.
The most common side effects of VIDEX EC include:
- diarrhea
- stomach pain
- nausea
- vomiting
- headache
- rash
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of VIDEX EC. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VIDEX EC?
- Store VIDEX EC capsules in a tightly closed container between 59° F to 86° F (15° C to 30° C)
- Safely throw away any unused VIDEX EC
Keep VIDEX EC and all medicines out of the reach of children and pets.
General Information about the safe and effective use of VIDEX EC
Avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VIDEX EC for a condition for which it was not prescribed. Do not give VIDEX EC to other people, even if they have the same symptoms as you have. It may harm them.
Do not keep medicine that is out of date or that you no longer need. Dispose of unused medicines through community take-back disposal programs when available or place VIDEX EC in an unrecognizable closed container in the household trash.
This Medication Guide summarizes the most important information about VIDEX EC. If you would like more information about VIDEX EC, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about VIDEX EC that is written for health professionals. For more information, go to www.bms.com/products/Pages/prescribing.aspx or call 1-800-321-1335.
What are the ingredients in VIDEX EC?
Active Ingredients: didanosine
Inactive Ingredients:
Carboxymethylcellulose sodium 12, diethyl phthalate, methacrylic acid copolymer, sodium hydroxide, sodium starch glycolate, talc, gelatin, and titanium dioxide.
VIDEX® EC and Zerit® are registered trademarks of Bristol-Myers Squibb Company. All other trademarks are the property of their respective owners.
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
Product of Japan
This Medication Guide has been approved by the U.S. Food and Drug Administration.
1251544A7
Rev August 2013
---------------------------------------------
REPRESENTATIVE PACKAGING
See HOW SUPPLIED section for a complete list of available packages of VIDEX EC.
PRINCIPAL DISPLAY PANEL - 125 mg Label
30 Capsules NDC 0087-6671-17
125 mg
VIDEX®EC
(didanosine, USP)
delayed-release capsules
enteric-coated beadlets
Detach and dispense
the accompanying
Medication Guide
to the patient.
Rx only

PRINCIPAL DISPLAY PANEL - 200 mg Label
30 Capsules NDC 0087-6672-17
200 mg
VIDEX®EC
(didanosine, USP)
delayed-release capsules
enteric-coated beadlets
Detach and dispense
the accompanying
Medication Guide
to the patient.
Rx only

PRINCIPAL DISPLAY PANEL - 250 mg Label
30 Capsules NDC 0087-6673-17
250 mg
VIDEX®EC
(didanosine, USP)
delayed-release capsules
enteric-coated beadlets
Detach and dispense
the accompanying
Medication Guide
to the patient.
Rx only

PRINCIPAL DISPLAY PANEL - 400 mg Label
30 Capsules NDC 0087-6674-17
400 mg
VIDEX®EC
(didanosine, USP)
delayed-release capsules
enteric-coated beadlets
Detach and dispense
the accompanying
Medication Guide
to the patient.
Rx only

VIDEX ECdidanosine CAPSULE, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
VIDEX ECdidanosine CAPSULE, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
VIDEX ECdidanosine CAPSULE, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
VIDEX ECdidanosine CAPSULE, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||