Vimovo
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VIMOVO safely and effectively. See full prescribing information for VIMOVO. VIMOVO (naproxen and esomeprazole magnesium) delayed release tablets, for oral useInitial U.S. Approval: 2010BOXED WARNING WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISKS See full prescribing information for complete boxed warning Cardiovascular Risk Naproxen, a component of VIMOVO, may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (5.1) VIMOVO is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery. (4, 5.1) Gastrointestinal Risk NSAIDs, including naproxen, a component of VIMOVO, cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal (GI) events. (5.4) RECENT MAJOR CHANGES Warnings and Precautions, Renal Effects (5.6) 03/2014 INDICATIONS AND USAGERelief of signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID associated gastric ulcers (1)DOSAGE AND ADMINISTRATIONOne tablet twice daily. Use the lowest effective dose. Should be avoided in moderate/severe renal insufficiency or in severe hepatic insufficiency. Consider dose reduction in mild/moderate hepatic insufficiency (2)DOSAGE FORMS AND STRENGTHSDelayed release tablets: 375 mg/20 mg or 500 mg/20 mg of naproxen and esomeprazole magnesium (3)CONTRAINDICATIONS Known hypersensitivity to any component of VIMOVO or substituted benzimidazoles (4) History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (4, 5.8, 5.9, 5.13) Use during the peri-operative period in the setting of coronary artery bypass graft (CABG) surgery (4, 5.1) WARNINGS AND PRECAUTIONS Serious and potentially fatal cardiovascular (CV) thrombotic events, myocardial infarction, and stroke. Patients with known CV disease/risk factors may be at greater risk (5.1) New onset or worsening of pre-existing hypertension. Blood pressure should be monitored closely during treatment with VIMOVO (5.2, 7.1, 7.6) Congestive heart failure and edema. VIMOVO should be used with caution in patients with fluid retention or heart failure (5.3) Serious gastrointestinal (GI) adverse events, which can be fatal. The risk is greater in patients with a prior history of ulcer disease or GI bleeding, and in patients at high risk for GI events, especially the elderly. VIMOVO should be used with caution in these patients (5.4, 8.5) Symptomatic response to esomeprazole does not preclude the presence of gastric malignancy (5.4) Atrophic gastritis has been noted on biopsy with long-term omeprazole therapy (5.4) Treatment should be withdrawn when active and clinically significant bleeding from any source occurs (5.5) Renal papillary necrosis and other renal injury with long-term use. Use VIMOVO with caution in the elderly, those with impaired renal function, hypovolemia, salt depletion, heart failure, liver dysfunction, and those taking diuretics, ACE-inhibitors or angiotensin II receptor antagonists. Not recommended for patients with moderate or severe renal impairment (2, 5.6, 5.7, 7.1, 7.6, 8.7) Anaphylactic reactions. Do not use VIMOVO in patients with the aspirin triad (5.8) Serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which can be fatal and can occur without warning. Discontinue VIMOVO at first appearance of skin rash or any other sign of hypersensitivity (5.9) Elevated liver enzymes and, rarely, severe hepatic reactions. Discontinue use immediately if abnormal liver enzymes persist or worsen (5.11, 8.6, 12.3) Should be avoided in patients with severe hepatic impairment (e.g., Child-Pugh C) (2, 5.11, 8.6, 12.3) Proton pump inhibitor (PPI) therapy may be associated with increased risk of Clostridium difficile associated diarrhea. (5.16) Avoid concomitant use of esomeprazole with clopidogrel (5.17) Long-term and multiple daily dose PPI therapy is associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine (5.18) Interactions with diagnostic investigations for Neuroendocrine Tumors: Increases in intragastric pH may result in hypergastrinemia, enterochromaffin-like cell hyperplasia, and increased Chromogranin A levels which may interfere with diagnostic investigations for neuroendocrine tumors. (5.20) Hypomagnesemia has been reported rarely with prolonged treatment with PPIs (5.21) Avoid concomitant use of VIMOVO with St John's Wort or rifampin due to the potential reduction in esomeprazole levels. (5.22, 7.16) Fetal toxicity: avoid drug starting at 30 weeks gestation (5.10, 8.1) Side EffectsMost common adverse reactions in clinical trials (>5%): erosive gastritis, dyspepsia, gastritis, diarrhea, gastric ulcer, upper abdominal pain, nausea (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Horizon Pharma USA, Inc. at 1-866-479-6742 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Concomitant use of NSAIDs may reduce the antihypertensive effect of ACE Inhibitors, angiotensin II receptor antagonists, diuretics, and beta-blockers (7.1, 7.6, 7.11) As with all NSAIDs caution is advised when cyclosporin is co-administered because of the increased risk of nephrotoxicity. (7.4) Tacrolimus: Concomitant administration of esomeprazole, a component of VIMOVO, and tacrolimus may increase the serum levels of tacrolimus. (7.5) Concomitant use of NSAIDs increases lithium plasma levels (7.7) Methotrexate: VIMOVO may increase serum levels of methotrexate (7.8) Concomitant use of VIMOVO with warfarin may result in increased risk of bleeding complications. Monitor for increases in INR and prothrombin time (7.9) Esomeprazole inhibits gastric acid secretion and may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (e.g., ketoconazole, iron salts, erlotinib, and digoxin). Patients treated with VIMOVO and digoxin may need to be monitored for increases in digoxin toxicity (7.14) Clopidogrel: esomeprazole, a component of VIMOVO, decreases exposure to the active metabolite of clopidogrel. (7.16, 12.3) USE IN SPECIFIC POPULATIONS Hepatic Insufficiency: VIMOVO should be avoided in patients with severe hepatic insufficiency (2, 4, 5.11, 8.6, 12.3) Renal Insufficiency: VIMOVO is not recommended in patients with moderate or severe renal insufficiency (2, 5.6, 5.7, 8.7, 12.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 VIMOVO INDICATIONS AND USAGE
- 2 VIMOVO DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 VIMOVO CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Cardiovascular Thrombotic Events
- 5.2 Hypertension
- 5.3 Congestive Heart Failure and Edema
- 5.4 Gastrointestinal Effects — Risk of Ulceration, Bleeding, and Perforation
- 5.5 Active Bleeding
- 5.6 Renal Effects
- 5.7 Advanced Renal Disease
- 5.8 Anaphylactic Reactions
- 5.9 Skin Reactions
- 5.10 Fetal Toxicity
- 5.11 Hepatic Effects
- 5.12 Hematological Effects
- 5.13 Pre-existing Asthma
- 5.14 Concomitant NSAID Use
- 5.15 Corticosteroid Treatment
- 5.16associated diarrhea
- 5.17 Interaction with Clopidogrel
- 5.18 Bone Fracture
- 5.19 Masking of Inflammation and Fever
- 5.20 Laboratory Tests
- 6 VIMOVO ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 7.1 ACE-inhibitors/Angiotensin II Receptor Antagonists
- 7.2 Aspirin
- 7.3 Cholestyramine
- 7.4 Cyclosporin
- 7.5 Tacrolimus
- 7.6 Diuretics
- 7.7 Lithium
- 7.8 Methotrexate
- 7.9 Anticoagulants
- 7.10 Selective Serotonin Reuptake Inhibitors (SSRIs)
- 7.11 Other Information Concerning Drug Interactions
- 7.12 Interactions With Investigations of Neuroendocrine Tumors
- 7.13 Drug/Laboratory Test Interaction
- 7.14 Interactions Related to Absorption
- 7.15 Antiretroviral Agents
- 7.16 Effects on Hepatic Metabolism/Cytochrome P-450 pathways
- 7.17 Other Pharmacokinetic-based Interactions
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 VIMOVO DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
Cardiovascular Risk
- Non-Steroidal Anti-inflammatory Drugs (NSAIDs), a component of VIMOVO, may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk [see Warnings and Precautions (5.1) ].
- VIMOVO is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4), and Warnings and Precautions (5.1) ].
Gastrointestinal Risk
- NSAIDs, including naproxen, a component of VIMOVO, cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events [see Warnings and Precautions (5.4) ].
1 INDICATIONS AND USAGE
VIMOVO is a combination product that contains naproxen and esomeprazole. It is indicated for the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers. VIMOVO is not recommended for initial treatment of acute pain because the absorption of naproxen is delayed compared to absorption from other naproxen-containing products. Controlled studies do not extend beyond 6 months.
2 DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of VIMOVO and other treatment options before deciding to use VIMOVO. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. VIMOVO does not allow for administration of a lower daily dose of esomeprazole. If a dose of esomeprazole lower than a total daily dose of 40 mg is more appropriate, a different treatment should be considered.
Rheumatoid Arthritis, Osteoarthritis and Ankylosing Spondylitis
The dosage is one tablet twice daily of VIMOVO 375 mg naproxen and 20 mg of esomeprazole or 500 mg naproxen and 20 mg of esomeprazole.
The tablets are to be swallowed whole with liquid. Do not split, chew, crush or dissolve the tablet. VIMOVO is to be taken at least 30 minutes before meals.
Geriatric Patients
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Use caution when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly use the lowest effective dose [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.3) ].
Patients With Moderate to Severe Renal Impairment
Naproxen-containing products are not recommended for use in patients with moderate to severe or severe renal impairment (creatinine clearance <30 mL/min) [see Warnings and Precautions (5.6, 5.7) and Use in Specific Populations (8.7) ].
Hepatic Insufficiency
Monitor patients with mild to moderate hepatic impairment closely and consider a possible dose reduction based on the naproxen component of VIMOVO.
VIMOVO should be avoided in patients with severe hepatic impairment [see Warnings and Precautions (5.11), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ].
Pediatric Patients
The safety and efficacy of VIMOVO in children younger than 18 years has not been established. VIMOVO is therefore not recommended for use in children.
3 DOSAGE FORMS AND STRENGTHS
Oval, yellow, delayed release tablets for oral administration containing either:
- 375 mg enteric coated naproxen and 20 mg esomeprazole (as magnesium trihydrate) tablets printed with 375/20 in black, or
- 500 mg enteric coated naproxen and 20 mg esomeprazole (as magnesium trihydrate) tablets printed with 500/20 in black.
4 CONTRAINDICATIONS
VIMOVO is contraindicated in patients with known hypersensitivity to naproxen, esomeprazole magnesium, substituted benzimidazoles, or to any of the excipients.
VIMOVO is contraindicated in patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.8, 5.13) ]. Hypersensitivity reactions, e.g., angioedema and anaphylactic reaction/shock, have been reported with esomeprazole use.
VIMOVO is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1) ].
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDS, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use.
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10–14 days following CABG surgery found an increased incidence of myocardial infarction and stroke [see Contraindications (4) ].
5.2 Hypertension
NSAIDs, including naproxen, a component of VIMOVO, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy [see Drug Interactions (7.1, 7.6) ].
5.3 Congestive Heart Failure and Edema
Fluid retention, edema, and peripheral edema have been observed in some patients taking NSAIDs and should be used with caution in patients with fluid retention or heart failure.
5.4 Gastrointestinal Effects — Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including naproxen, a component of VIMOVO, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. While VIMOVO has been shown to significantly decrease the occurrence of gastric ulcers compared to naproxen alone, ulceration and associated complications can still occur.
These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3–6 months, and in about 2–4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. The utility of periodic laboratory monitoring has not been demonstrated, nor has it been adequately assessed.
VIMOVO should be prescribed with caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk of developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants or antiplatelets (including low-dose aspirin), longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients, and therefore special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID or NSAID-containing product, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Epidemiological studies of the case-control and cohort design have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. In two studies, concurrent use of an NSAID, COX-2 inhibitor, or aspirin potentiated the risk of bleeding [see Drug Interactions (7.2, 7.10) ]. Although these studies focused on upper gastrointestinal bleeding, bleeding at other sites cannot be ruled out.
NSAIDs should be given with care to patients with a history of inflammatory bowel disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated.
Gastrointestinal symptomatic response to therapy with VIMOVO does not preclude the presence of gastric malignancy.
Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole, of which esomeprazole is an enantiomer and a component of VIMOVO.
5.5 Active Bleeding
When active and clinically significant bleeding from any source occurs in patients receiving VIMOVO, the treatment should be withdrawn.
5.6 Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, hypovolemia, heart failure, liver dysfunction, salt depletion, those taking diuretics, ACE inhibitors, or angiotensin II receptor antagonists and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
5.7 Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of VIMOVO in patients with advanced renal disease. Therefore, treatment with VIMOVO is not recommended in these patients with advanced renal disease. If VIMOVO therapy must be initiated, close monitoring of the patient's renal function is advisable [see Dosage and Administration (2), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3) ].
5.8 Anaphylactic Reactions
Anaphylactic reactions may occur in patients without known prior exposure to either component of VIMOVO. NSAIDs should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs [see Contraindications (4) ]. Emergency help should be sought in cases where an anaphylactic reaction occurs. Anaphylactic reactions, like anaphylaxis, may have a fatal outcome.
5.9 Skin Reactions
NSAIDs can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
5.10 Fetal Toxicity
Starting at 30 weeks gestation, VIMOVO, as with other NSAID-containing products, should be avoided because it may cause premature closure of the ductus arteriosus [see Use in Specific Populations (8.1) ].
5.11 Hepatic Effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including naproxen, a component of VIMOVO. Hepatic abnormalities may be the result of hypersensitivity rather than direct toxicity. These laboratory abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes, have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of more severe hepatic reaction while on therapy with VIMOVO.
If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), VIMOVO should be discontinued.
Chronic alcoholic liver disease and probably other diseases with decreased or abnormal plasma proteins (albumin) reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased. Caution is advised when high doses are required and some adjustment of dosage may be required in these patients. It is prudent to use the lowest effective dose for the shortest possible duration of adequate treatment.
VIMOVO should be avoided in patients with severe hepatic impairment [see Dosage and Administration (2) , Use in Specific Populations (8.6), and Clinical Pharmacology (12.3) ].
5.12 Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving VIMOVO who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants or antiplatelets, should be carefully monitored.
5.13 Pre-existing Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, VIMOVO should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with pre-existing asthma.
5.14 Concomitant NSAID Use
VIMOVO contains naproxen as one of its active ingredients. It should not be used with other naproxen-containing products since they all circulate in the plasma as the naproxen anion.
The concomitant use of VIMOVO with any dose of a non-aspirin NSAID should be avoided due to the potential for increased risk of adverse reactions.
5.15 Corticosteroid Treatment
VIMOVO cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids and the patient should be observed closely for any evidence of adverse effects, including adrenal insufficiency and exacerbation of symptoms of arthritis.
5.16associated diarrhea
Published observational studies suggest that proton pump inhibitor (PPI) therapy like VIMOVO may be associated with an increased risk of Clostridium difficile associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions (6.2) ].
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. [see Dosage and Administration (2) ].
5.17 Interaction with Clopidogrel
Avoid concomitant use of esomeprazole with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as esomeprazole, that inhibit CYP2C19 activity. Concomitant use of clopidogrel with 40 mg esomeprazole reduces the pharmacological activity of clopidogrel. When using esomeprazole, a component of VIMOVO, consider alternative anti-platelet therapy [see Drug Interactions (7.16) and Pharmacokinetics (12.3) ]
5.18 Bone Fracture
Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to the established treatment guidelines. [see Dosage and Administration (2) and Adverse Reactions (6.2) ].
VIMOVO (a combination PPI/NSAID) is approved for use twice a day and does not allow for administration of a lower daily dose of the PPI. [see Dosage and Administration (2) ].
5.19 Masking of Inflammation and Fever
The pharmacological activity of VIMOVO in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, noninflammatory painful conditions.
5.20 Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, VIMOVO should be discontinued.
Patients with initial hemoglobin values of 10 g or less who are to receive long-term therapy should have hemoglobin values determined periodically.
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Providers should temporarily stop esomeprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Clinical Pharmacology (12.2) ].
5.21 Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions (6.2) ].
5.22 Concomitant use of St John's Wort or Rifampin with VIMOVO
Drugs that induce CYP2C19 or CYP3A4 (such as St John's Wort or rifampin) can substantially decrease esomeprazole concentrations. Avoid concomitant use of VIMOVO with St John's Wort or rifampin [see Drug Interactions (7.16) ].
5.23 Concomitant use of VIMOVO with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration a temporary withdrawal of the PPI may be considered in some patients [see Drug Interactions (7.8) ].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reactions reported below are specific to the clinical trials with VIMOVO. See also the full prescribing information for naproxen and esomeprazole magnesium products.
The safety of VIMOVO was evaluated in clinical studies involving 2317 patients (aged 27 to 90 years) and ranging from 3-12 months. Patients received either 500 mg/20 mg of VIMOVO twice daily (n=1157), 500 mg of enteric-coated naproxen twice daily (n=426), or placebo (n=246). The average number of VIMOVO doses taken over 12 months was 696±44.
The table below lists all adverse reactions, regardless of causality, occurring in >2% of patients receiving VIMOVO from two clinical studies (Study 1 and Study 2). Both of these studies were randomized, multi-center, double-blind, parallel studies. The majority of patients were female (67%), white (86%). The majority of patients were 50-69 years of age (83%). Approximately one quarter were on low-dose aspirin.
| Preferred term (sorted by SOC) | VIMOVO 500 mg/20 mg twice daily (n=428) % |
EC-Naproxen 500 mg twice daily (n=426) % |
|---|---|---|
| Gastrointestinal Disorders | ||
| Gastritis Erosive | 19 | 38 |
| Dyspepsia | 18 | 27 |
| Gastritis | 17 | 14 |
| Diarrhea | 6 | 5 |
| Gastric Ulcer | 6 | 24 |
| Abdominal Pain Upper | 6 | 9 |
| Nausea | 5 | 5 |
| Hiatus Hernia | 4 | 6 |
| Abdominal Distension | 4 | 4 |
| Flatulence | 4 | 3 |
| Esophagitis | 4 | 8 |
| Constipation | 3 | 3 |
| Abdominal pain | 2 | 2 |
| Erosive Duodenitis | 2 | 12 |
| Abdominal pain lower | 2 | 3 |
| Duodenitis | 1 | 7 |
| Gastritis hemorrhagic | 1 | 2 |
| Gastroesophageal reflux disease | <1 | 4 |
| Duodenal ulcer | <1 | 5 |
| Erosive esophagitis | <1 | 6 |
| Infections and infestations | ||
| Upper respiratory tract infection | 5 | 4 |
| Bronchitis | 2 | 2 |
| Urinary tract infection | 2 | 1 |
| Sinusitis | 2 | 2 |
| Nasopharyngitis | <1 | 2 |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 1 | 2 |
| Nervous system disorders | ||
| Headache | 3 | 1 |
| Dysgeusia | 2 | 1 |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 2 | 3 |
In Study 1 and Study 2, patients taking VIMOVO had fewer premature discontinuations due to adverse reactions compared to patients taking enteric-coated naproxen alone (7.9% vs. 12.5% respectively). The most common reasons for discontinuations due to adverse events in the VIMOVO treatment group were upper abdominal pain (1.2%, n=5), duodenal ulcer (0.7%, n=3) and erosive gastritis (0.7%, n=3). Among patients receiving enteric-coated naproxen, the most common reasons for discontinuations due to adverse events were duodenal ulcer 5.4% (n=23), dyspepsia 2.8% (n=12) and upper abdominal pain 1.2% (n=5). The proportion of patients discontinuing treatment due to any upper gastrointestinal adverse events (including duodenal ulcers) in patients treated with VIMOVO was 4% compared to 12% for patients taking enteric-coated naproxen.
The table below lists all adverse reactions, regardless of causality, occurring in >2% of patients from 2 clinical studies conducted in patients with osteoarthritis of the knee (Study 3 and Study 4).
| Preferred term (sorted by SOC) | VIMOVO 500 mg/20 mg twice daily (n=490) % |
Placebo (n=246) % |
|---|---|---|
| Gastrointestinal Disorders | ||
| Dyspepsia | 8 | 12 |
| Diarrhea | 6 | 4 |
| Abdominal Pain Upper | 4 | 3 |
| Constipation | 4 | 1 |
| Nausea | 4 | 4 |
| Nervous System Disorders | ||
| Dizziness | 3 | 2 |
| Headache | 3 | 5 |
| General disorders and administration site conditions | ||
| Peripheral edema | 3 | 1 |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 1 | 3 |
| Infections and infestations | ||
| Sinusitis | 1 | 2 |
The percentage of subjects who withdrew from the VIMOVO treatment group in these studies due to treatment-emergent adverse events was 7%. There were no preferred terms in which more than 1% of subjects withdrew from any treatment group.
The long-term safety of VIMOVO was evaluated in an open-label clinical trial of 239 patients, of which 135 patients received 500 mg/20 mg of VIMOVO for 12 months. There were no differences in frequency or types of adverse reactions seen in the long-term safety study compared to shorter-term treatment in the randomized controlled studies.
6.2 Postmarketing Experience
Naproxen
The following adverse reactions have been identified during post-approval use of naproxen. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system:
Body as a Whole: anaphylactic reactions, angioneurotic edema, menstrual disorders, pyrexia (chills and fever)
Cardiovascular: congestive heart failure, vasculitis, hypertension, pulmonary edema
Gastrointestinal: gastrointestinal bleeding and/or perforation, hematemesis, pancreatitis, vomiting, colitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn's disease), nonpeptic gastrointestinal ulceration, ulcerative stomatitis, esophagitis, peptic ulceration
Hepatobiliary: jaundice, abnormal liver function tests, hepatitis (some cases have been fatal)
Hemic and Lymphatic: eosinophilia, leukopenia, melena, thrombocytopenia, agranulocytosis, granulocytopenia, hemolytic anemia, aplastic anemia
Metabolic and Nutritional: hyperglycemia, hypoglycemia
Nervous System: inability to concentrate, depression, dream abnormalities, insomnia, malaise, myalgia, muscle weakness, aseptic meningitis, cognitive dysfunction, convulsions
Respiratory: eosinophilic pneumonitis, asthma
Dermatologic: alopecia, urticaria, skin rashes, toxic epidermal necrolysis, erythema multiforme, erythema nodosum, fixed drug eruption, lichen planus, pustular reaction, systemic lupus erythematoses, bullous reactions, including Stevens-Johnson syndrome, photosensitive dermatitis, photosensitivity reactions, including rare cases resembling porphyria cutanea tarda (pseudoporphyria) or epidermolysis bullosa. If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored.
Special Senses: hearing impairment, corneal opacity, papillitis, retrobulbar optic neuritis, papilledema
Urogenital: glomerular nephritis, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, renal disease, renal failure, renal papillary necrosis, raised serum creatinine
Reproduction (female): infertility
Esomeprazole
The following adverse reactions have been identified during post-approval use of esomeprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system:
Blood and Lymphatic: agranulocytosis, pancytopenia
Eye: blurred vision
Gastrointestinal: pancreatitis; stomatitis; microscopic colitis
Hepatobiliary: hepatic failure, hepatitis with or without jaundice
Immune System: anaphylactic reaction/shock
Infections and Infestations: GI candidiasis, Clostridium difficile associated diarrhea
Metabolism and Nutritional Disorders: hypomagnesemia
Musculoskeletal and Connective Tissue: muscular weakness, myalgia, bone fracture
Nervous System: hepatic encephalopathy, taste disturbance
Psychiatric: aggression, agitation, depression, hallucination
Renal and Urinary: interstitial nephritis
Reproductive System and Breast : gynecomastia
Respiratory, Thoracic, and Mediastinal: bronchospasm
Skin and Subcutaneous Tissue: alopecia, erythema multiforme, hyperhidrosis, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal)
7 DRUG INTERACTIONS
Several studies conducted with VIMOVO have shown no interaction between the two components, naproxen and esomeprazole.
7.1 ACE-inhibitors/Angiotensin II Receptor Antagonists
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors and angiotensin II receptor antagonists. NSAIDs may also increase the risk of renal impairment associated with the use of ACE-inhibitors or angiotensin II receptor antagonists. Monitor renal function closely in patients taking VIMOVO concomitantly with ACE-inhibitors or angiotensin II receptor antagonists who are elderly, volume-depleted, or with impaired renal function [see Use in Specific Populations (8.5, 8.7)].
7.2 Aspirin
VIMOVO can be administered with low-dose aspirin (≤325 mg/day) therapy. The concurrent use of aspirin and VIMOVO may increase the risk of serious adverse events [see Warnings and Precautions (5.1, 5.4), Adverse Reactions (6), and Clinical Studies (14) ].
When naproxen is administered with doses of aspirin (>1 gram/day), its protein binding is reduced. The clinical significance of this interaction is not known. However, as with other NSAIDs, concomitant administration of naproxen and aspirin is not generally recommended because of the potential of increased adverse effects.
7.3 Cholestyramine
As with other NSAIDs, concomitant administration of cholestyramine can delay the absorption of naproxen.
7.4 Cyclosporin
As with all NSAIDs caution is advised when cyclosporin is co-administered because of the increased risk of nephrotoxicity.
7.5 Tacrolimus
Concomitant administration of esomeprazole, a component of VIMOVO, and tacrolimus may increase the serum levels of tacrolimus.
7.6 Diuretics
Clinical studies, as well as postmarketing observations, have shown that NSAIDs can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely both for signs of renal failure, as well as to monitor to assure diuretic efficacy [see Warnings and Precautions (5.6, 5.7) ].
7.7 Lithium
NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
7.8 Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. NSAIDs have been reported to reduce the tubular secretion of methotrexate in an animal model. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of PPIs and methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate. However, no formal drug interaction studies of methotrexate with PPIs have been conducted [see Warnings and Precautions (5.23) ].
7.9 Anticoagulants
Naproxen decreases platelet aggregation and may prolong bleeding time. In addition, because warfarin and NSAIDs are highly protein bound, the free fraction of warfarin and naproxen may increase substantially in some patients.
Concomitant use of VIMOVO and anticoagulants (such as warfarin, dicumarol and heparin) may result in increased risk of bleeding complications.
The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
Post-marketing reports of changes in prothrombin measures have been reported among patients on concomitant warfarin and esomeprazole therapy. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
7.10 Selective Serotonin Reuptake Inhibitors (SSRIs)
There is an increased risk of gastrointestinal bleeding when selective serotonin reuptake inhibitors (SSRIs) are combined with NSAIDs including COX-2 selective inhibitors. Caution should be used when NSAIDs are administered concomitantly with SSRIs [see Warnings and Precautions (5.4) ].
7.11 Other Information Concerning Drug Interactions
Naproxen is highly bound to plasma albumin; it thus has a theoretical potential for interaction with other albumin-bound drugs such as sulphonylureas, hydantoins, and other NSAIDs. Patients simultaneously receiving VIMOVO and a hydantoin, sulphonamide or sulphonylurea should be observed for adjustment of dose if required.
Naproxen and other NSAIDs can reduce the antihypertensive effect of propranolol and other beta-blockers.
Probenecid given concurrently increases naproxen anion plasma levels and extends its plasma half-life significantly.
7.12 Interactions With Investigations of Neuroendocrine Tumors
Drug-induced decrease in gastric acidity results in enterochromaffin-like cell hyperplasia and increased Chromogranin A levels which may interfere with investigations for neuroendocrine tumors [see Warnings and Precautions (5.20) and Clinical Pharmacology (12.2) ].
7.13 Drug/Laboratory Test Interaction
Naproxen may decrease platelet aggregation and prolong bleeding time. This effect should be kept in mind when bleeding times are determined.
The administration of naproxen may result in increased urinary values for 17-ketogenic steroids because of an interaction between the drug and/or its metabolites with m-di-nitrobenzene used in this assay. Although 17-hydroxy-corticosteroid measurements (Porter-Silber test) do not appear to be artifactually altered, it is suggested that therapy with naproxen be temporarily discontinued 72 hours before adrenal function tests are performed if the Porter-Silber test is to be used.
Naproxen may interfere with some urinary assays of 5-hydroxy indoleacetic acid (5HIAA).
7.14 Interactions Related to Absorption
Esomeprazole inhibits gastric acid secretion. Therefore, esomeprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability. Like with other drugs that decrease the intragastric acidity, the absorption of drugs such as ketoconazole, iron salts and erlotinib can decrease, while the absorption of drugs such as digoxin can increase during treatment with esomeprazole. Concomitant treatment with omeprazole (20 mg daily) and digoxin in healthy subjects increased the bioavailability of digoxin by 10% (30% in two subjects). Esomeprazole is an enantiomer of omeprazole. Coadministration of digoxin with esomeprazole is expected to increase the systemic exposure of digoxin. Therefore, patients may need to be monitored for increases in digoxin toxicity when digoxin is taken concomitantly with esomeprazole.
7.15 Antiretroviral Agents
Concomitant use of atazanavir and nelfinavir with proton pump inhibitors such as esomeprazole is not recommended. Co-administration of atazanavir with proton pump inhibitors is expected to substantially decrease atazanavir plasma concentrations and thereby reduce its therapeutic effect.
Omeprazole, the racemate of esomeprazole, has been reported to interact with some antiretroviral drugs. The clinical importance and the mechanisms behind these interactions are not always known. Increased gastric pH during omeprazole treatment may change the absorption of the antiretroviral drug. Other possible interaction mechanisms are via CYP2C19. For some antiretroviral drugs, such as atazanavir and nelfinavir, decreased serum levels have been reported when given together with omeprazole. Following multiple doses of nelfinavir (1250 mg, twice daily) and omeprazole (40 mg once a day), AUC was decreased by 36% and 92%, Cmax by 37% and 89% and Cmin by 39% and 75% respectively for nelfinavir and main oxidative metabolite, hydroxy-t-butylamide (M8). Following multiple doses of atazanavir (400 mg, once a day) and omeprazole (40 mg, once a day, 2 hr before atazanavir), AUC was decreased by 94%, Cmax by 96%, and Cmin by 95%. Concomitant administration with omeprazole and drugs such as atazanavir and nelfinavir is therefore not recommended. For other antiretroviral drugs, such as saquinavir, elevated serum levels have been reported with an increase in AUC by 82% in Cmax by 75% and in Cmin by 106% following multiple dosing of saquinavir/ritonavir (1000/100 mg) twice a day for 15 days with omeprazole 40 mg once a day co-administered on days 11 to 15. Therefore, clinical and laboratory monitoring for saquinavir toxicity is recommended during concurrent use with esomeprazole. Dose reduction of saquinavir should be considered from the safety perspective for individual patients. There are also some antiretroviral drugs of which unchanged serum levels have been reported when given with omeprazole.
7.16 Effects on Hepatic Metabolism/Cytochrome P-450 pathways
Esomeprazole is extensively metabolized in the liver by CYP2C19 and CYP3A4.
In vitro and in vivo studies have shown that esomeprazole is not likely to inhibit CYPs 1A2, 2A6, 2C9, 2D6, 2E1 and 3A4. No clinically relevant interactions with drugs metabolized by these CYP enzymes would be expected. Drug interaction studies have shown that esomeprazole does not have any clinically significant interactions with phenytoin, warfarin, quinidine, clarithromycin or amoxicillin.
However, post-marketing reports of changes in prothrombin measures have been received among patients on concomitant warfarin and esomeprazole therapy. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
Esomeprazole may potentially interfere with CYP2C19, the major esomeprazole metabolizing enzyme. Co-administration of esomeprazole 30 mg and diazepam, a CYP2C19 substrate, resulted in a 45% decrease in clearance of diazepam.
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of esomeprazole 40 mg results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition. Avoid concomitant administration of esomeprazole with clopidogrel. When using esomeprazole, a component of VIMOVO, consider use of alternative anti-platelet therapy [see Pharmacokinetics (12.3) ].
Concomitant administration of esomeprazole and a combined inhibitor of CYP2C19 and CYP3A4, such as voriconazole, may result in more than doubling of the esomeprazole exposure. Dose adjustment of esomeprazole is not normally required. Omeprazole acts as an inhibitor of CYP2C19. Omeprazole, given in doses of 40 mg daily for one week to 20 healthy subjects in cross-over study, increased Cmax and AUC of cilostazol by 18% and 26% respectively. Cmax and AUC of one of its active metabolites, 3,4-dihydrocilostazol, which has 4-7 times the activity of cilostazol, were increased by 29% and 69% respectively. Co-administration of cilostazol with esomeprazole is expected to increase concentrations of cilostazol and its above mentioned active metabolite. Therefore a dose reduction of cilostazol from 100 mg twice daily to 50 mg twice daily should be considered.
Drugs known to induce CYP2C19 or CYP3A4 (such as rifampin) may lead to decreased esomeprazole serum levels. Omeprazole, of which esomeprazole is an enantiomer, has been reported to interact with St. John's Wort, an inducer of CYP3A4. In a cross-over study in 12 healthy male subjects, St John's Wort (300 mg three times daily for 14 days) significantly decreased the systemic exposure of omeprazole in CYP2C19 poor metabolizers (Cmax and AUC decreased by 37.5% and 37.9%, respectively) and extensive metabolizers (Cmax and AUC decreased by 49.6% and 43.9%, respectively). Avoid concomitant use of St. John's Wort or rifampin with VIMOVO.
7.17 Other Pharmacokinetic-based Interactions
Co-administration of oral contraceptives, diazepam, phenytoin, or quinidine does not seem to change the pharmacokinetic profile of esomeprazole.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C prior to 30 weeks gestation; Category D starting 30 weeks gestation.
Risk Summary
There are no adequate and well-controlled studies with VIMOVO in pregnant women. VIMOVO contains naproxen and esomeprazole magnesium. Esomeprazole is the S-isomer of omeprazole. Available epidemiologic data fail to demonstrate an increased risk of major congenital malformations or other adverse pregnancy outcomes with first trimester omeprazole use. However, starting at 30 weeks gestation VIMOVO, as with other NSAID-containing products, should be avoided because NSAIDs can cause premature closure of the ductus arteriosus. Animal reproduction studies with naproxen conducted in rats and rabbits at 0.23 and 0.27 times the human systemic exposure showed no evidence of impaired fertility or harm to the fetus. Teratogenicity was not observed in animal reproduction studies with esomeprazole magnesium at doses about 57 times and 35 times a human dose of 40 mg (based on body surface area) in rats and rabbits, respectively. However, changes in bone morphology and physeal dysplasia were observed in offspring of rats dosed through most of pregnancy and lactation at doses equal to or greater than approximately 33.6 times an oral human dose of 40 mg (see Animal Data). Because of the observed effect at high doses of esomeprazole magnesium on developing bone in rat studies, Vimovo should be used prior to 30 weeks gestation only if the potential benefit justifies the potential risk to the fetus. Starting at 30 weeks gestation, if this drug is used, the patient should be apprised of the potential hazard to the fetus.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
There is some evidence to suggest that when inhibitors of prostaglandin synthesis, such as an NSAID, naproxen, a component of VIMOVO, are used to delay preterm labor there is an increased risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus and intracranial hemorrhage. Naproxen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction and abnormal prostaglandin E levels in preterm infants.
Labor or Delivery
Naproxen-containing products are not recommended in labor and delivery because, through its prostaglandin synthesis inhibitory effect, naproxen may adversely affect fetal circulation and inhibit uterine contractions, thus increasing the risk of uterine hemorrhage. In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred.
Human Data
Esomeprazole is the S-isomer of omeprazole. Four epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy with the frequency of abnormalities among infants of women exposed to H2-receptor antagonists or other controls.
A population-based retrospective cohort epidemiological study from the Swedish Medical Birth Registry, covering approximately 99% of pregnancies, from 1995-99, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. The number of infants exposed in utero to omeprazole that had any malformation, low birth weight, low Apgar score, or hospitalization was similar to the number observed in this population. The number of infants born with ventricular septal defects and the number of stillborn infants was slightly higher in the omeprazole-exposed infants than the expected number in this population.
A population-based retrospective cohort study covering all live births in Denmark from 1996-2009, reported on 1,800 live births whose mothers used omeprazole during the first trimester of pregnancy and 837, 317 live births whose mothers did not use any proton pump inhibitor. The overall rate of birth defects in infants born to mothers with first trimester exposure to omeprazole was 2.9% and 2.6% in infants born to mothers not exposed to any proton pump inhibitor during the first trimester.
A retrospective cohort study reported on 689 pregnant women exposed to either H2-blockers or omeprazole in the first trimester (134 exposed to omeprazole) and 1,572 pregnant women unexposed to either during the first trimester. The overall malformation rate in offspring born to mothers with first trimester exposure to omeprazole, an H2-blocker, or were unexposed was 3.6%, 5.5%, and 4.1% respectively.
A small prospective observational cohort study followed 113 women exposed to omeprazole during pregnancy (89% first trimester exposures). The reported rate of major congenital malformations was 4% in the omeprazole group, 2% in controls exposed to non-teratogens, and 2.8% in disease-paired controls. Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight were similar among the groups.
Several studies have reported no apparent adverse short-term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Animal Data
There are no reproduction studies in animals with VIMOVO. Reproduction studies with naproxen have been performed in rats at 20 mg/kg/day (125 mg/m2/day, 0.23 times the human systemic exposure), rabbits at 20 mg/kg/day (220 mg/m2/day, 0.27 times the human systemic exposure), and mice at 170 mg/kg/day (510 mg/m2/day, 0.28 times the human systemic exposure) with no evidence of impaired fertility or harm to the fetus due to the drug. Reproduction studies have been performed with esomeprazole magnesium in rats at oral doses up to 280 mg/kg/day (about 57 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 35 times an oral human dose of 40 mg on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to esomeprazole magnesium.
A pre- and postnatal developmental toxicity study in rats with additional endpoints to evaluate bone development were performed with esomeprazole magnesium at oral doses of 14 to 280 mg/kg/day (about 3.4 to 57 times a daily human dose of 40 mg on a body surface area basis). Neonatal/early postnatal (birth to weaning) survival was decreased at doses equal to or greater than 138 mg/kg/day (about 33 times an oral human dose of 40 mg on a body surface area basis). Body weight and body weight gain were reduced and neurobehavioral or general developmental delays in the immediate post-weaning timeframe were evident at doses equal to or greater than 69 mg /kg/day (about 16.8 times an oral human dose of 40 mg on a body surface area basis). In addition, decreased femur length, width and thickness of cortical bone, decreased thickness of the tibial growth plate and minimal to mild bone marrow hypocellularity were noted at doses equal to or greater than 14 mg/kg/day (about 3.4 times a daily human dose of 40 mg on a body surface area basis). Physeal dysplasia in the femur was observed in offspring of rats treated with oral doses of esomeprazole magnesium at doses equal to or greater than 138 mg/kg/day (about 33.6 times the daily human dose of 40 mg on a body surface area basis).
Effects on maternal bone were observed in pregnant and lactating rats in the pre- and postnatal toxicity study when esomeprazole magnesium was administered at oral doses of 14 to 280 mg /kg/day (about 3.4 to 57 times an oral human dose of 40 mg on a body surface area basis). When rats were dosed from gestational day 7 through weaning on postnatal day 21, a statistically significant decrease in maternal femur weight of up to 14% (as compared to placebo treatment) was observed at doses equal to or greater than 138 mg/kg/day (about 33.6 times an oral human dose of 40 mg on a body surface area basis).
A pre- and postnatal development study in rats with esomeprazole strontium (using equimolar doses compared to esomeprazole magnesium study) produced similar results in dams and pups as described above.
8.3 Nursing Mothers
VIMOVO is likely present in human milk. The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma. Esomeprazole is the S-isomer of omeprazole and limited data indicate that maternal doses of omeprazole 20 mg daily produce low levels in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VIMOVO and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition. Exercise caution when administering VIMOVO to a nursing woman.
8.4 Pediatric Use
The safety and efficacy of VIMOVO has not been established in pediatric patients younger than 18 years.
Juvenile Animal Data
In a juvenile rat toxicity study, esomeprazole was administered with both magnesium and strontium salts at oral doses about 34 to 57 times a daily human dose of 40 mg based on body surface area. Increases in death were seen at the high dose, and at all doses of esomeprazole, there were decreases in body weight, body weight gain, femur weight and femur length, and decreases in overall growth [see Nonclinical Toxicology (13.2)].
8.5 Geriatric Use
Of the total number of patients who received VIMOVO (n=1157) in clinical trials, 387 were ≥65 years of age, of which 85 patients were 75 years and over. No meaningful differences in efficacy or safety were observed between these subjects and younger subjects [see Adverse Reactions (6) ].
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose [see Dosage and Administration (2) and Clinical Pharmacology (12.3) ].
Experience indicates that geriatric patients may be particularly sensitive to certain adverse effects of NSAIDs. Elderly or debilitated patients seem to tolerate peptic ulceration or bleeding less well when these events do occur. Most spontaneous reports of fatal GI events are in the geriatric population [see Warnings and Precautions (5.4) ].
Naproxen is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Geriatric patients may be at a greater risk for the development of a form of renal toxicity precipitated by reduced prostaglandin formation during administration of NSAIDs [see Warnings and Precautions (5.6, 5.7) ].
8.6 Hepatic Insufficiency
VIMOVO should be avoided in patients with severe hepatic impairment because naproxen may increase the risk of renal failure or bleeding and esomeprazole doses should not exceed 20 mg daily in these patients [see Dosage and Administration (2), Warnings and Precautions (5.11 ), and Clinical Pharmacology (12.3) ].
8.7 Renal Insufficiency
Naproxen-containing products, including VIMOVO, are not recommended for use in patients with advanced renal disease [see Dosage and Administration (2), and Warnings and Precautions (5.6, 5.7) ].
8.8 Females and Males of Reproductive Potential
Infertility
Data from several small studies in humans and from studies in animals indicate that NSAIDs, including naproxen, may be associated with a reversible delay in ovulation. Therefore, in women who have difficulties conceiving, or who are undergoing investigation of infertility, use of VIMOVO is not recommended.
10 OVERDOSAGE
There is no clinical data on overdosage with VIMOVO.
Overdosage of naproxen:
Significant naproxen overdosage may be characterized by lethargy, dizziness, drowsiness, epigastric pain, abdominal discomfort, heartburn, indigestion, nausea, transient alterations in liver function, hypoprothrombinemia, renal dysfunction, metabolic acidosis, apnea, disorientation or vomiting. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression, and coma may occur, but are rare. Anaphylactic reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose. A few patients have experienced convulsions, but it is not clear whether or not these were drug-related. It is not known what dose of the drug would be life threatening. The oral LD50 of the drug is 543 mg/kg in rats, 1234 mg/kg in mice, 4110 mg/kg in hamsters, and greater than 1000 mg/kg in dogs.
Patients should be managed by symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. Hemodialysis does not decrease the plasma concentration of naproxen because of the high degree of its protein binding. Activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose. Forced diuresis, alkalinization of urine or hemoperfusion may not be useful due to high protein binding.
Overdosage of esomeprazole:
A single oral dose of esomeprazole at 510 mg/kg (about 103 times the human dose on a body surface area basis) was lethal to rats. The major signs of acute toxicity were reduced motor activity, changes in respiratory frequency, tremor, ataxia, and intermittent clonic convulsions.
The symptoms described in connection with deliberate esomeprazole overdose (limited experience of doses in excess of 240 mg/day) are transient. Single doses of 80 mg of esomeprazole were uneventful. Reports of overdosage with omeprazole in humans may also be relevant. Doses ranged up to 2,400 mg (120 times the usual recommended clinical dose). Manifestations were variable, but included confusion, drowsiness, blurred vision, tachycardia, nausea, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen in normal clinical experience (see omeprazole package insert - Adverse Reactions). No specific antidote for esomeprazole is known. Since esomeprazole is extensively protein bound, it is not expected to be removed by dialysis. In the event of overdosage, treatment should be symptomatic and supportive.
If overexposure occurs, call the Poison Control Center at 1-800-222-1222.
11 DESCRIPTION
The active ingredients of VIMOVO are naproxen which is a NSAID and esomeprazole magnesium which is a Proton Pump Inhibitor (PPI).
VIMOVO is available as an oval, yellow, multi-layer, delayed release tablet combining an enteric coated naproxen core and an immediate release esomeprazole magnesium layer surrounding the core. Each strength contains either 375 mg of naproxen and 20 mg of esomeprazole (present as 22.3 mg esomeprazole magnesium trihydrate) or 500 mg of naproxen and 20 mg of esomeprazole (present as 22.3 mg esomeprazole magnesium trihydrate) for oral administration. The inactive ingredients are carnauba wax, colloidal silicon dioxide, croscarmellose sodium, iron oxide yellow, glyceryl monostearate, hypromellose, iron oxide black, magnesium stearate, methacrylic acid copolymer dispersion, methylparaben, polysorbate 80, polydextrose, polyethylene glycol, povidone, propylene glycol, propylparaben, titanium dioxide, and triethyl citrate.
The chemical name for naproxen is (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid. Naproxen has the following structure:
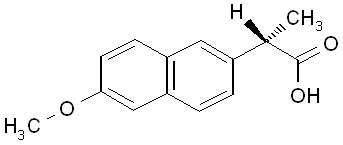
Naproxen has a molecular weight of 230.26 and a molecular formula of C14H14O3.
Naproxen is an odorless, white to off-white crystalline substance. It is lipid soluble, practically insoluble in water at low pH and freely soluble in water at high pH. The octanol/water partition coefficient of naproxen at pH 7.4 is 1.6 to 1.8.
The chemical name for esomeprazole is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl) magnesium trihydrate. Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers. Its molecular formula is (C17H18N3O3S)2Mg × 3 H2O with molecular weight of 767.2 as a trihydrate and 713.1 on an anhydrous basis. The structural formula is:
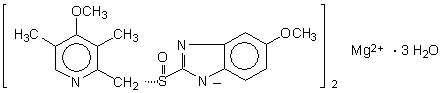
The magnesium salt is a white to slightly colored crystalline powder. It contains 3 moles of water of solvation and is slightly soluble in water.
The stability of esomeprazole magnesium is a function of pH; it rapidly degrades in acidic media, but it has acceptable stability under alkaline conditions. At pH 6.8 (buffer), the half-life of the magnesium salt is about 19 hours at 25°C and about 8 hours at 37°C.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VIMOVO consists of an immediate-release esomeprazole magnesium layer and an enteric-coated naproxen core. As a result, esomeprazole is released first in the stomach, prior to the dissolution of naproxen in the small intestine. The enteric coating prevents naproxen release at pH levels below 5.5.
Naproxen is a NSAID with analgesic and antipyretic properties. The mechanism of action of the naproxen anion, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. Esomeprazole is protonated and converted in the acidic compartment of the parietal cell forming the active inhibitor, the achiral sulphenamide. By acting specifically on the proton pump, esomeprazole blocks the final step in acid production, thus reducing gastric acidity. This effect is dose-related up to a daily dose of 20 to 40 mg and leads to inhibition of gastric acid secretion.
12.2 Pharmacodynamics
Antisecretory Activity
The effect of VIMOVO on intragastric pH was determined in 25 healthy volunteers in one study. Three VIMOVO combinations (naproxen 500 mg combined with either esomeprazole 10, 20, or 30 mg) were administered twice daily over 9 days. The results are shown in the following table:
| Naproxen 500 mg combined with esomeprazole | |||
|---|---|---|---|
| 10 mg | 20 mg | 30 mg | |
| LS Mean (SE) | |||
| % Time Gastric pH >4  |
41.1 (3.0) | 71.5 (3.0) | 76.8 (3.0) |
| Coefficient of variation | 55% | 18% | 16% |
Serum Gastrin Effects
The effect of esomeprazole on serum gastrin concentrations was evaluated in approximately 2,700 patients in clinical trials up to 8 weeks and in over 1,300 patients for up to 6-12 months. The mean fasting gastrin level increased in a dose-related manner. This increase reached a plateau within two to three months of therapy and returned to baseline levels within four weeks after discontinuation of therapy.
Increased gastrin causes enterochromaffin-like cell hyperplasia and increased serum Chromogranin A (CgA) levels. The increased CgA levels may cause false positive results in diagnostic investigations for neuroendocrine tumors. Healthcare providers should temporarily stop esomeprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high.
Enterochromaffin-like (ECL) Cell Effects
In over 1,000 patients treated with esomeprazole (10, 20 or 40 mg/day) up to 6-12 months, the prevalence of ECL cell hyperplasia increased with time and dose. No patient developed ECL cell carcinoids, dysplasia, or neoplasia in the gastric mucosa.
Endocrine Effects
Esomeprazole had no effect on thyroid function when given in oral doses of 20 or 40 mg for 4 weeks. Other effects of esomeprazole on the endocrine system were assessed using omeprazole studies. Omeprazole given in oral doses of 30 or 40 mg for 2 to 4 weeks had no effect on carbohydrate metabolism, circulating levels of parathyroid hormone, cortisol, estradiol, testosterone, prolactin, cholecystokinin or secretin.
Effects on Gastrointestinal Microbial Ecology
Decreased gastric acidity due to any means including proton pump inhibitors, increases gastric counts of bacteria normally present in the gastrointestinal tract. Treatment with proton pump inhibitors may lead to slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter and, in hospitalized patients, possibly also Clostridium difficile.
12.3 Pharmacokinetics
Absorption
Naproxen
At steady state following administration of VIMOVO twice daily, peak plasma concentrations of naproxen are reached on average 3 hours following both the morning and the evening dose.
Bioequivalence between VIMOVO and enteric-coated naproxen, based on both area under the plasma concentration-time curve (AUC) and maximum plasma concentration (Cmax) of naproxen, has been demonstrated for both the 375 mg and 500 mg doses.
Naproxen is absorbed from the gastrointestinal tract with an in vivo bioavailability of 95%.
Steady-state levels of naproxen are reached in 4 to 5 days.
Esomeprazole
Following administration of VIMOVO twice daily, esomeprazole is rapidly absorbed with peak plasma concentration reached within on average, 0.43 to 1.2 hours, following the morning and evening dose on both the first day of administration and at steady state. The peak plasma concentrations of esomeprazole are higher at steady state compared to on first day of dosing of VIMOVO.
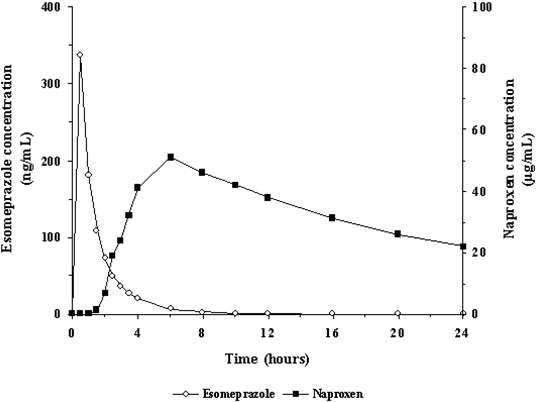
Figure 1 represents the pharmacokinetics of naproxen and esomeprazole following administration of VIMOVO 500 mg/20 mg.
Figure 1: Mean plasma concentrations of naproxen and esomeprazole following single dose administration of VIMOVO (500mg/20 mg)
Food effect
Administration of VIMOVO together with high-fat food in healthy volunteers does not affect the extent of absorption of naproxen but significantly prolongs tmax by 10 hours and decreases peak plasma concentration (Cmax) by about 12%.
Administration of VIMOVO together with high-fat food in healthy volunteers delays tmax of esomeprazole by 1 hour and significantly reduces the extent of absorption, resulting in 52% and 75% reductions of area under the plasma concentration versus time curve (AUC) and peak plasma concentration (Cmax), respectively.
Administration of VIMOVO 30 minutes before high-fat food intake in healthy volunteers does not affect the extent of absorption of naproxen but delays the absorption by about 4 hours and decreases peak plasma concentration (Cmax) by about 17%, but has no significant effect on the rate or extent of esomeprazole absorption compared to administration under fasted conditions [see Dosage and Administration (2) ].
Administration of VIMOVO 60 minutes before high-fat food intake in healthy volunteers has no effect on the rate and extent of naproxen absorption; however, increases the esomeprazole AUC by 25% and Cmax by 50% compared to administration under fasted conditions. This increase in esomeprazole Cmax does not raise a safety issue since the approved dosing regimen of esomeprazole at 40 mg QD would result in higher Cmax [see Dosage and Administration (2) ].
Therefore, VIMOVO should be taken at least 30 minutes before the meal.
Distribution
Naproxen
Naproxen has a volume of distribution of 0.16 L/kg. At therapeutic levels naproxen is greater than 99% albumin-bound. At doses of naproxen greater than 500 mg/day there is less than proportional increase in plasma levels due to an increase in clearance caused by saturation of plasma protein binding at higher doses (average trough Css 36.5, 49.2 and 56.4 mg/L with 500, 1000 and 1500 mg daily doses of naproxen, respectively). The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma [see Use in Specific Populations (8.3) ].
Esomeprazole
The apparent volume of distribution at steady state in healthy subjects is approximately 16L. Esomeprazole is 97% plasma protein bound.
Metabolism
Naproxen
Naproxen is extensively metabolized in the liver by the cytochrome P450 system (CYP), CYP2C9 and CYP1A2, to 6-0-desmethyl naproxen. Neither the parent drug nor the metabolites induce metabolizing enzymes. Both naproxen and 6-0-desmethyl naproxen are further metabolized to their respective acylglucuronide conjugated metabolites. Consistent with the half-life of naproxen, the area under the plasma concentration time curve increases with repeated dosing of VIMOVO twice daily.
Esomeprazole
Esomeprazole is extensively metabolized in the liver by the CYP enzyme system. The major part of the metabolism of esomeprazole is dependent on the polymorphic CYP2C19, responsible for the formation of the hydroxyl- and desmethyl metabolites of esomeprazole. The remaining part is dependent on another specific isoform CYP3A4, responsible for the formation of esomeprazole sulphone, the main metabolite in plasma. The major metabolites of esomeprazole have no effect on gastric acid secretion.
The area under the plasma esomeprazole concentration-time curve increases with repeated administration of VIMOVO. This increase is dose-dependent and results in a non-linear dose-AUC relationship after repeated administration. An increased absorption of esomeprazole with repeated administration of VIMOVO probably also contributes to the time-and dose-dependency.
Excretion
Naproxen
Following administration of VIMOVO twice daily, the mean elimination half-life for naproxen is approximately 15 hours following the evening dose, with no change with repeated dosing.
The clearance of naproxen is 0.13 mL/min/kg. Approximately 95% of the naproxen from any dose is excreted in the urine, primarily as naproxen (<1%), 6-0-desmethyl naproxen (<1%) or their conjugates (66% to 92%). Small amounts, 3% or less of the administered dose, are excreted in the feces. In patients with renal failure, metabolites may accumulate [see Warnings and Precautions (5.6, 5.7) ].
Esomeprazole
Following administration of VIMOVO twice daily, the mean elimination half-life of esomeprazole is approximately 1 hour following both the morning and evening dose on day 1, with a slightly longer elimination half-life at steady state (1.2-1.5 hours).
Almost 80% of an oral dose of esomeprazole is excreted as metabolites in the urine, the remainder in the feces. Less than 1% of the parent drug is found in the urine.
Concomitant Use with Clopidogrel
Results from a crossover study in healthy subjects have shown a pharmacokinetic interaction between clopidogrel (300 mg loading dose/75 mg daily maintenance dose) and esomeprazole (40 mg p.o. once daily) when co-administered for 30 days. Exposure to the active metabolite of clopidogrel was reduced by 35% to 40% over this time period. Pharmacodynamic parameters were also measured and demonstrated that the change in inhibition of platelet aggregation was related to the change in the exposure to clopidogrel active metabolite.
Special Populations
Geriatric Patients
There is no specific data on the pharmacokinetics of VIMOVO in patients over age 65.
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly, although the unbound fraction is <1% of the total naproxen concentration. Unbound trough naproxen concentrations in elderly subjects have been reported to range from 0.12% to 0.19% of total naproxen concentration, compared with 0.05% to 0.075% in younger subjects. The clinical significance of this finding is unclear, although it is possible that the increase in free naproxen concentration could be associated with an increase in the rate of adverse events per a given dosage in some elderly patients [see Adverse Reactions (6) and Use in Specific Populations (8.5) ].
The AUC and Cmax values of esomeprazole were slightly higher (25% and 18%, respectively) in the elderly as compared to younger subjects at steady state. Dosage adjustment for the esomeprazole component based on age is not necessary.
Race
Pharmacokinetic differences due to race have not been studied for naproxen.
Approximately 3% of Caucasians and 15 to 20% of Asians lack a functional CYP2C19 enzyme and are called poor metabolizers. In these individuals the metabolism of esomeprazole is probably mainly catalyzed by CYP3A4. After repeated once-daily administration of 40 mg esomeprazole, the mean area under the plasma concentration-time curve was approximately 100% higher in poor metabolizers than in subjects having a functional CYP2C19 enzyme (extensive metabolizers).
Hepatic Insufficiency
The pharmacokinetics of VIMOVO or naproxen have not been determined in subjects with hepatic impairment.
In patients with severe hepatic impairment, VIMOVO should be avoided due to increase of risk of NSAID associated bleeding and/or renal failure associated with naproxen.
Chronic alcoholic liver disease and probably also other forms of cirrhosis reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased. The implication of this finding for the naproxen component of VIMOVO dosing is unknown but it is prudent to use the lowest effective dose.
The AUCs of esomeprazole in patients with severe hepatic insufficiency (Child Pugh Class C) have been shown to be 2-3 times higher than in patients with normal liver function. For this reason, it has been recommended that esomeprazole doses not exceed 20 mg daily in patients with severe hepatic impairment. However, there is no dose adjustment necessary for patients with Child Pugh Class A and B for the esomeprazole component of VIMOVO. There is no VIMOVO dosage form that contains less than 20 mg esomeprazole for twice daily dosing [see Dosage and Administration (2), Warnings and Precautions (5.11) , and Use in Specific Populations (8.6) ].
Renal Insufficiency
The pharmacokinetics of VIMOVO or naproxen have not been determined in subjects with renal impairment.
Given that naproxen, its metabolites and conjugates are primarily excreted by the kidney, the potential exists for naproxen metabolites to accumulate in the presence of renal insufficiency. Elimination of naproxen is decreased in patients with severe renal impairment. Naproxen-containing products, including VIMOVO, is not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance <30 ml/min) [see Dosage and Administration (2), Warnings and Precautions (5.6 , 5.7) , and Use in Specific Populations (8.7) ].
No studies have been performed with esomeprazole in patients with decreased renal function. Since the kidney is responsible for the excretion of the metabolites of esomeprazole but not for the elimination of the parent compound, the metabolism of esomeprazole is not expected to be changed in patients with impaired renal function.
Gender
The AUC and Cmax values of esomeprazole were slightly higher (13%) in females than in males at steady state. Dosage adjustment for the esomeprazole component based on gender is not necessary.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Naproxen
A 2-year study was performed in rats to evaluate the carcinogenic potential of naproxen at rat doses of 8, 16, and 24 mg/kg/day (50, 100, and 150 mg/m2). The maximum dose used was 0.28 times the highest recommended human dose. No evidence of tumorigenicity was found.
Esomeprazole
The carcinogenic potential of esomeprazole was assessed using omeprazole studies, of which esomeprazole is an enantiomer. In two 24-month oral carcinogenicity studies in rats, omeprazole at daily doses of 1.7, 3.4, 13.8, 44 and 140.8 mg/kg/day (about 0.35 to 28 times the human dose of 40 mg/day expressed on a body surface area basis) produced gastric ECL cell carcinoids in a dose-related manner in both male and female rats; the incidence of this effect was markedly higher in female rats, which had higher blood levels of omeprazole. Gastric carcinoids seldom occur in the untreated rat. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (about 2.8 times the human dose of 40 mg/day on a body surface area basis) for 1 year, then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of 1 year (94% treated vs 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs 26%) but still showed more hyperplasia in the treated group. Gastric adenocarcinoma was seen in one rat (2%). No similar tumor was seen in male or female rats treated for 2 years. For this strain of rat no similar tumor has been noted historically, but a finding involving only one tumor is difficult to interpret. A 78-week mouse carcinogenicity study of omeprazole did not show increased tumor occurrence, but the study was not conclusive.
Esomeprazole was negative in the Ames mutation test, in the in vivo rat bone marrow cell chromosome aberration test, and the in vivo mouse micronucleus test. Esomeprazole, however, was positive in the in vitro human lymphocyte chromosome aberration test. Omeprazole was positive in the in vitro human lymphocyte chromosome aberration test, the in vivo mouse bone marrow cell chromosome aberration test, and the in vivo mouse micronucleus test.
The potential effects of esomeprazole on fertility and reproductive performance were assessed using omeprazole studies. Omeprazole at oral doses up to 138 mg/kg/day in rats (about 28 times the human dose of 40 mg/day on a body surface area basis) was found to have no effect on reproductive performance of parental animals.
13.2 Animal Toxicology and/or Pharmacology
Naproxen
Reproduction studies have been performed in rats at 20 mg/kg/day (125 mg/m2/day, 0.23 times the maximum recommended human dose), rabbits at 20 mg/kg/day (220 mg/m2/day, 0.27 times the maximum recommended human dose), and mice at 170 mg/kg/day (510 mg/m2/day, 0.28 times the maximum recommended human dose) with no evidence of impaired fertility or harm to the fetus due to the drug. However, animal reproduction studies are not always predictive of human response.
Esomeprazole – Reproduction Studies
Reproduction studies have been performed in rats at oral doses up to 280 mg/kg/day (about 57 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 35 times an oral human dose of 40 mg on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to esomeprazole [see Pregnancy (8.1) ].
Esomeprazole – Juvenile Animal Data
A 28-day toxicity study with a 14-day recovery phase was conducted in juvenile rats with esomeprazole magnesium at doses of 70 to 280 mg /kg/day (about 17 to 57 times a daily oral human dose of 40 mg on a body surface area basis). An increase in the number of deaths at the high dose of 280 mg /kg/day was observed when juvenile rats were administered esomeprazole magnesium from postnatal day 7 through postnatal day 35. In addition, doses equal to or greater than 140 mg/kg/day (about 34 times a daily oral human dose of 40 mg on a body surface area basis), produced treatment-related decreases in body weight (approximately 14%) and body weight gain, decreases in femur weight and femur length, and affected overall growth. Comparable findings described above have also been observed in this study with another esomeprazole salt, esomeprazole strontium, at equimolar doses of esomeprazole.
14 CLINICAL STUDIES
Two randomized, multi-center, double-blind trials (Study 1 and Study 2) compared the incidence of gastric ulcer formation in 428 patients taking VIMOVO and 426 patients taking enteric-coated naproxen. Subjects were at least 18 years of age with a medical condition expected to require daily NSAID therapy for at least 6 months, and, if less than 50 years old, with a documented history of gastric or duodenal ulcer within the past 5 years. The majority of patients were female (67%), white (86%). The majority of patients were 50-69 years of age (83%). Approximately one quarter were on low-dose aspirin.
Studies 1 and 2 showed that VIMOVO given as 500 mg/20 mg twice daily statistically significantly reduced the 6-month cumulative incidence of gastric ulcers compared to enteric-coated naproxen 500 mg twice daily (see Table 4).
Approximately a quarter of the patients in Studies 1 and 2 were taking concurrent low-dose aspirin (≤ 325 mg daily). The results for this subgroup analysis in patients who used aspirin were consistent with the overall findings of the study.
The results at one month, three months, and six months are presented in Table 4.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| VIMOVO N=218 number (%) |
EC-naproxen N=216 number (%) |
VIMOVO N=210 number (%) |
EC-naproxen N=210 number (%) |
|
| 0-1 Month | 3 (1.4) | 28 (13.0) | 4 (1.9) | 21 (10.0) |
| 0-3 Months | 4 (1.8) | 42 (19.4) | 10 (4.8) | 37 (17.6) |
0-6 Months
 |
9 (4.1) | 50 (23.1) | 15 (7.1) | 51 (24.3) |
In these trials, patients receiving VIMOVO had a mean duration of therapy of 152 days compared to 124 days in patients receiving enteric-coated naproxen alone. A higher proportion of patients taking EC-naproxen (12%) discontinued the study due to upper GI adverse events (including duodenal ulcers) compared to VIMOVO (4%) in both trials [see Adverse Reactions (6) ].
The efficacy of VIMOVO in treating the signs and symptoms of osteoarthritis was established in two 12-week randomized, double-blind, placebo-controlled trials in patients with osteoarthritis (OA) of the knee. In these two trials, patients were allowed to remain on low-dose aspirin for cardioprophylaxis. VIMOVO was given as 500 mg/20 mg twice daily. In each trial, patients receiving VIMOVO had significantly better results compared to patients receiving placebo as measured by change from baseline of the WOMAC pain subscale and the WOMAC physical function subscale and a Patient Global Assessment Score.
Based on studies with enteric-coated naproxen, improvement in patients treated for rheumatoid arthritis was demonstrated by a reduction in joint swelling, a reduction in duration of morning stiffness, a reduction in disease activity as assessed by both the investigator and patient, and by increased mobility as demonstrated by a reduction in walking time. In patients with osteoarthritis, the therapeutic action of naproxen has been shown by a reduction in joint pain or tenderness, an increase in range of motion in knee joints, increased mobility as demonstrated by a reduction in walking time, and improvement in capacity to perform activities of daily living impaired by the disease. In patients with ankylosing spondylitis, naproxen has been shown to decrease night pain, morning stiffness and pain at rest.
16 HOW SUPPLIED/STORAGE AND HANDLING
VIMOVO 375 mg/20 mg tablets are oval, yellow film-coated tablets printed with 375/20 in black ink, supplied as:
| NDC 75987-031-04 | Bottles of 60 tablets |
VIMOVO 500 mg/20 mg tablets are oval, yellow film-coated tablets printed with 500/20 in black ink, supplied as:
| NDC 75987-030-04 | Bottles of 60 tablets |
Storage: Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Store in the original container and keep the bottle tightly closed to protect from moisture. Dispense in a tight container if package is subdivided.
17 PATIENT COUNSELING INFORMATION
"See FDA-Approved Medication Guide"
Patients should be informed of the following before initiating therapy with VIMOVO and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
1. VIMOVO, like other NSAID-containing products, may cause serious cardiovascular side effects, such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up [see Warnings and Precautions (5.1) ].
2. VIMOVO has been developed with esomeprazole to decrease incidence of ulceration from naproxen. NSAIDs, including naproxen, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up [see Warnings and Precautions (5.4) ].
3. VIMOVO, like other NSAID-containing products, can cause serious skin side effects such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible [see Warnings and Precautions (5.9) ].
4. Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
5. Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy [see Contraindications (4) and Warnings and Precautions (5.11) ].
6. Patients should be informed of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help [see Warnings and Precautions (5.8) ].
7. Starting at 30 weeks gestation, VIMOVO, as with other NSAIDs, should be avoided because it may cause premature closure of the ductus arteriosus [see Warnings and Precautions (5.10) and Use in Specific Populations (8.1) ].
8. Caution should be exercised by patients whose activities require alertness if they experience drowsiness, dizziness, vertigo or depression during therapy with VIMOVO.
9. Patients should be instructed to tell their physicians if they have a history of asthma or aspirin-sensitive asthma because the use of NSAIDs in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Patients with this form of aspirin sensitivity should be instructed not to take VIMOVO. Patients with preexisting asthma should be instructed to seek immediate medical attention if their asthma worsens after taking VIMOVO [see Warnings and Precautions (5.8, 5.13) ].
10. Antacids may be used while taking VIMOVO.
11. VIMOVO tablets should be swallowed whole with liquid. Tablets should not be split, chewed, crushed or dissolved. VIMOVO tablets should be taken at least 30 minutes before meals [see Dosage and Administration (2) ].
12. Advise patients to immediately report and seek care for diarrhea that does not improve. This may be a sign of Clostridium difficile associated diarrhea [see Warnings and Precautions (5.16) ].
13. Advise patients to immediately report and seek care for any cardiovascular or neurological symptoms including palpitations, dizziness, seizures, and tetany as these may be signs of hypomagnesemia [see Warnings and Precautions (5.21) ].
VIMOVO is a trademark of the AstraZeneca group of companies.
The VIMOVO trademark has been licensed to Horizon Pharma for use in the US.
Other trademarks are the property of AstraZeneca respective companies.
Distributed by: Horizon Pharma USA, Inc., Deerfield, IL 60015
Rev. 03/2014
MEDICATION GUIDE
VIMOVO® (vi-moh-voh)
(naproxen and esomeprazole magnesium)
delayed release tablets
Read this Medication Guide before you start taking VIMOVO and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about VIMOVO?
VIMOVO, which contains naproxen [a nonsteroidal anti-inflammatory drug (NSAID)] and esomeprazole magnesium, may increase the chance of a heart attack or stroke that can lead to death. This chance increases:
- with longer use of NSAID medicines
- in people who have heart disease
NSAID-containing medicines, such as VIMOVO, should never be used right before or after a heart surgery called a coronary artery bypass graft (CABG).
NSAID-containing medicines, such as VIMOVO, can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
- can happen without warning symptoms
- may cause death
The chance of a person getting an ulcer or bleeding increases with:
- taking medicines called steroid hormones (corticosteroids) and blood thinners (anticoagulants)
- longer use
- smoking
- drinking alcohol
- older age
- having poor health
NSAID medicines should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
- different types of arthritis
- menstrual cramps and other types of short-term pain
Who should not take a Non–Steroidal Anti–Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
- if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- for pain right before or after heart bypass surgery
Tell your healthcare provider:
- about all of your medical conditions
- about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
- if you are pregnant. NSAID medicines should not be used past 30 weeks of pregnancy.
- if you are breastfeeding. Talk to your healthcare provider.
What are the possible side effects of Non–Steroidal Anti–Inflammatory Drugs (NSAIDs)?
| Serious side effects include: | Other side effects include: |
| heart attack | stomach pain |
| stroke | constipation |
| high blood pressure | diarrhea |
| heart failure from body swelling (fluid retention) | gas |
| kidney problems including kidney failure | heartburn |
| bleeding and ulcers in the stomach and intestine | nausea |
| low red blood cells (anemia) | vomiting |
| life-threatening skin reactions | dizziness |
| life-threatening allergic reactions | |
| liver problems including liver failure | |
| asthma attacks in people who have asthma |
| Get emergency help right away if you have any of the following symptoms: | |
|
|
| Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms: | |
|
|
These are not all of the possible side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about Non–Steroidal Anti–Inflammatory Drugs (NSAIDs)
- Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some of these NSAID medicines are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
NSAID medicines that need a prescription
| Generic Name | TRADENAME |
|---|---|
| Celecoxib | Celebrex |
| Diclofenac | Zorvolex, Cataflam, Cambia, Voltaren, Voltaren gel, Arthrotec (combined with misoprostol), Flector, Zipsor, Pennsaid |
| Diflunisal | Dolobid |
| Etodolac | Lodine, Lodine XL |
| Fenoprofen | Nalfon, Nalfon 200 |
| Flurbiprofen | Ansaid |
| Ibuprofen | Motrin, Tab-Profen, Vicoprofen |
| Indomethacin | Tivorbex, Indocin, Indocin SR, Indo-Lemmon, Indomethagan |
| Ketoprofen | Oruvail, Nexcede |
| Ketorolac | Toradol, Sprix |
| Mefenamic Acid | Ponstel |
| Meloxicam | Mobic |
| Nabumetone | Relafen |
| Naproxen | Naprosyn, Anaprox, Anaprox DS, EC-Naprosyn, Naprelan, Naprapac (copackaged with lansoprazole), Treximet (combined with sumatriptan succinate), VIMOVO (combined with esomeprazole magnesium) |
| Oxaprozin | Daypro |
| Piroxicam | Feldene |
| Sulindac | Clinoril |
| Tolmetin | Tolectin, Tolectin DS, Tolectin 600 |
What other important information should I know about VIMOVO?
VIMOVO may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your healthcare provider.
VIMOVO can cause other serious side effects, including:
- Diarrhea. VIMOVO may increase your risk of getting severe diarrhea. This diarrhea may be caused by an infection (Clostridium difficile) in your intestines. Call your healthcare provider right away if you have watery stool, stomach pain, and fever that does not go away.
- Bone fractures. People who take multiple daily doses of proton pump inhibitor medicines for a long period of time (a year or longer) may have an increased risk of fractures of the hip, wrist, or spine. You should take VIMOVO exactly as prescribed, at the lowest dose possible for your treatment and for the shortest time needed. Talk to your healthcare provider about your risk of bone fracture if you take VIMOVO.
VIMOVO can have other serious side effects. See "What is the most important information I should know about VIMOVO?" and "What are the possible side effects of VIMOVO?"
What is VIMOVO?
VIMOVO contains 2 medicines: naproxen, a non-steroidal anti-inflammatory drug (NSAID) and esomeprazole magnesium, a proton pump inhibitor (PPI).
VIMOVO is a prescription medicine used to:
- relieve signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis
- decrease the risk of developing stomach (gastric) ulcers in people who are at risk of developing gastric ulcers with NSAIDs
It is not known if VIMOVO is safe or effective in children under the age of 18.
Who should not take VIMOVO?
Do not take VIMOVO:
- If you had an asthma attack, hives, or other allergic reaction after taking aspirin or other NSAID medicine.
- If you are allergic to any of the ingredients in VIMOVO. See the end of this Medication Guide for a complete list of ingredients in VIMOVO.
- If you are allergic to any other Proton Pump Inhibitor (PPI) medicine.
- For pain right before or after heart bypass surgery
What should I tell my healthcare provider before taking VIMOVO?
Before you take VIMOVO, tell your healthcare provider about all your medical conditions, including if you:
- have been told that you have low magnesium levels in your blood
- have liver or kidney problems
- have ulcerative colitis or Crohn's disease (inflammatory bowel disease or IBD)
- have any other medical conditions
- are pregnant or plan to become pregnant. See "What is the most important information I should know about VIMOVO?"
- are breastfeeding or plan to breastfeed. VIMOVO may pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take VIMOVO.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Since VIMOVO contains naproxen, talk to your healthcare provider before taking any other NSAID-containing products. Taking VIMOVO with other medicines can cause serious side effects. VIMOVO may affect the way other medicines work, and other medicines may affect how VIMOVO works.
Especially tell your healthcare provider if you take:
- steroid hormones (corticosteroids)
- St. John's Wort
- rifampin (Rifater, Rifamate, Rimactane, Rifadin)
- a medicine for high blood pressure or heart problems
- aspirin
- cholestyramine (Questran, Questran Light, Locholest, Locholest Light, Prevalite)
- cyclosporine (Gengraf, Neoral, Sandimmune) or tacrolimus (Prograf)
- a water pill (diuretic)
- lithium carbonate
- methotrexate
- a blood thinner medicine
- an antidepressant medicine
- atazanavir (Reyataz)
- ketoconazole (Nizoral)
- products that contain iron
- digoxin (Lanoxin)
- erlotinib (Tarceva)
- clopidogrel (Plavix)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take VIMOVO?
- Take VIMOVO exactly as your healthcare provider tells you to take it.
- Your healthcare provider may tell you to take Vitamin D and Calcium supplements during treatment with VIMOVO.
- Your healthcare provider will tell you how many VIMOVO to take and when to take them.
- Do not change your dose or stop VIMOVO without first talking to your healthcare provider.
- Take VIMOVO at least 30 minutes before a meal.
- Swallow VIMOVO tablets whole with liquid. Do not split, chew, crush or dissolve the VIMOVO tablet. Tell your healthcare provider if you cannot swallow the tablet whole. You may need a different medicine.
- You may use antacids while taking VIMOVO.
- If you forget to take a dose of VIMOVO, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take the next dose on time. Do not take 2 doses at one time to make up for a missed dose.
- If you take too much VIMOVO, tell your healthcare provider or go to the closest hospital emergency room right away. Symptoms that you have taken too much VIMOVO may include:
- feeling weak and tired
- dizziness
- feeling sleepy
- upper stomach-area pain or discomfort
- heartburn, indigestion, or nausea
- a change in breathing or you stop breathing
- vomiting
- bleeding
- movements of a body part that you cannot control
- coordination problems and decreased movement
- Your healthcare provider may do certain tests from time to time to check you for side effect of VIMOVO.
What should I avoid while taking VIMOVO?
VIMOVO can cause drowsiness, dizziness, or depression. You should not drive or do other activities that require you to be alert until you know how VIMOVO affects you.
What are the possible side effects of VIMOVO?
VIMOVO may cause serious side effects, including:
- See "What is the most important information I should know about VIMOVO?"
- High blood pressure.
- Heart problems such as congestive heart failure. Tell your healthcare provider about any swelling of your body, hands or feet, sudden weight gain or trouble breathing.
-
Active bleeding. Tell your healthcare provider if you have signs of active bleeding including:
- passing black sticky bowel movements (stools)
- having bloody diarrhea
- vomiting or coughing up blood or dark particles that look like coffee grounds
- Serious allergic reactions. Tell your healthcare provider or get medical help right away if you develop sudden wheezing, swelling of your lips, tongue, throat or body, rash, fainting or problems breathing or swallowing (severe allergic reaction).
-
Serious skin reactions. Tell your healthcare provider or get medical help right away if you develop:
- reddening of your skin with blisters or peeling
- blisters and bleeding of your lips, eye lids, mouth, nose, and genitals.
-
Liver problems. Tell your healthcare provider if you develop:
- yellowing of the skin or the whites of your eyes
- dark urine
- feel tired
- nausea
- right upper stomach area (abdominal) pain
- flu-like symptoms
- Chronic (lasting a long time) inflammation of the stomach lining (Atrophic Gastritis). Using VIMOVO for a long period of time may increase the risk of inflammation to your stomach lining. You may or may not have symptoms. Tell your healthcare provider if you have stomach pain, nausea, vomiting, or weight loss.
- Low magnesium levels in your body. Low magnesium can happen in some people who take a proton pump inhibitor medicine for at least 3 months. If low magnesium levels happen, it is usually after a year of treatment.
You may or may not have symptoms of low magnesium. Tell your healthcare provider right away if you have any of these symptoms:
- seizures
- dizziness
- abnormal or fast heart beat
- jitteriness
- jerking movements or shaking (tremors)
- muscle weakness
- spasms of the hands and feet
- cramps or muscle aches
- spasm of the voice box
Your healthcare provider may check the level of magnesium in your body before you start taking VIMOVO, during treatment, or if you will be taking VIMOVO for a long period of time.
The most common side effects of VIMOVO include:
- inflammation of the lining of the stomach, with or without loss of the protective layer of the stomach (erosive gastritis)
- indigestion
- diarrhea
- stomach ulcers
- upper stomach-area (abdominal) pain
- nausea
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of VIMOVO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store VIMOVO?
- Store VIMOVO at room temperature between 68°F to 77°F (20°C to 25°C)
- Keep VIMOVO in the original container and keep the bottle tightly closed.
- Keep VIMOVO dry.
Keep VIMOVO and all medicines out of the reach of children.
General information about VIMOVO
Medicines are sometimes prescribed for purposes other than those listed in this Medication Guide. Do not use VIMOVO for a condition for which it was not prescribed. Do not give VIMOVO to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about VIMOVO. If you would like more information, ask your healthcare provider. You can ask your healthcare provider or pharmacist for information that is written for healthcare professionals.
For more information, call 1-866-479-6742 or go to www.VIMOVO.com
What are the ingredients in VIMOVO?
Active ingredients: naproxen and esomeprazole magnesium
Inactive ingredients: carnauba wax, colloidal silicon dioxide, croscarmellose sodium, iron oxide yellow, glyceryl monostearate, hypromellose, iron oxide black, magnesium stearate, methacrylic acid copolymer dispersion, methylparaben, polysorbate 80, polydextrose, polyethylene glycol, povidone, propylene glycol, propylparaben, titanium dioxide, and triethyl citrate.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by: Horizon Pharma USA, Inc., Deerfield, IL 60015
Revised 03/2014
VIMOVO is a trademark of the AstraZeneca group of companies.
The VIMOVO trademark has been licensed to Horizon Pharma for use in the US.
Other trademarks are the property of AstraZeneca respective companies.
PRINCIPAL DISPLAY PANEL - 375 mg/20 mg Tablet Bottle Label
NDC 75987-031-04
60 tablets
Vimovo
®
(naproxen and esomeprazole
magnesium)
delayed release tablets
375 mg/20 mg*
*Each tablet contains 22.3 mg
esomeprazole magnesium, equivalent
to 20 mg of esomeprazole.
Dispense the enclosed Medication
Guide to each patient.
Rx only
HORIZON
PHARMA
PRINCIPAL DISPLAY PANEL - 500 mg/20 mg Tablet Bottle Label
NDC 75987-030-04
60 tablets
Vimovo
®
(naproxen and esomeprazole
magnesium)
delayed release tablets
500 mg/20 mg*
*Each tablet contains 22.3 mg
esomeprazole magnesium, equivalent
to 20 mg of esomeprazole.
Dispense the enclosed Medication
Guide to each patient.
Rx only
HORIZON
PHARMA
VimovoNaproxen and Esomeprazole Magnesium TABLET, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
VimovoNaproxen and Esomeprazole Magnesium TABLET, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||