VoSoL
ECR Pharmaceuticals Co., Inc.
Hi-Tech Pharmacal Co., Inc.
VōSoL Otic Solution(Acetic acid otic solution, USP)
FULL PRESCRIBING INFORMATION: CONTENTS*
- VOSOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- VOSOL INDICATIONS AND USAGE
- VOSOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PEDIATRIC USE
- VOSOL ADVERSE REACTIONS
- VOSOL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Rx only
VOSOL DESCRIPTION
VōSoL (Acetic acid otic solution, USP) is a solution containing acetic acid (2%) in a propylene glycol vehicle containing propylene glycol diacetate (3%), benzethonium chloride (0.02%), and sodium acetate (0.015%). The empirical formula for acetic acid is CH3COOH, with a molecular weight of 60.05. The structural formula is:
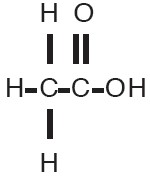
VōSoL is available as a nonaqueous otic solution buffered at pH 3 for use in the external ear canal.
CLINICAL PHARMACOLOGY
Acetic acid is antibacterial and antifungal; propylene glycol is hydrophilic and provides a low surface tension; benzethonium chloride is a surface active agent that promotes contact of the solution with tissues.
VOSOL INDICATIONS AND USAGE
For the treatment of superficial infections of the external auditory canal caused by organisms susceptible to the action of the antimicrobial.
VOSOL CONTRAINDICATIONS
Hypersensitivity to VōSoL or any of the ingredients. Perforated tympanic membrane is considered a contraindication to the use of any medication in the external ear canal.
WARNINGS
Discontinue promptly if sensitization or irritation occurs.
PRECAUTIONS
Transient stinging or burning may be noted occasionally when the solution is first instilled into the acutely inflamed ear.
PEDIATRIC USE
Safety and effectiveness in pediatric patients below the age of 3 years have not been established.
VOSOL ADVERSE REACTIONS
Stinging or burning may be noted occasionally; local irritation has occurred very rarely.
VOSOL DOSAGE AND ADMINISTRATION
Carefully remove all cerumen and debris to allow VōSoL to contact infected surfaces directly. To promote continuous contact, insert a wick of cotton saturated with VōSoL into the ear canal; the wick may also be saturated after insertion. Instruct the patient to keep the wick in for at least 24 hours and to keep it moist by adding 3 to 5 drops of VōSoL every 4 to 6 hours. The wick may be removed after 24 hours but the patient should continue to instill 5 drops of VōSoL 3 or 4 times daily thereafter, for as long as indicated. In pediatric patients, 3 to 4 drops may be sufficient due to the smaller capacity of the ear canal.
HOW SUPPLIED
VōSoL (Acetic acid otic solution, USP), containing acetic acid (2%), is available in 15 mL, measured-drop, safety-tip plastic bottles (NDC 0095-0202-15).
STORAGE
Store at 20°-25°C (68°-77°F).
Keep container tightly closed.
Rx Only
Manufactured For:
ECR Pharmaceuticals Co., Inc.
Richmond, Virginia 23255
Rev. 889:00 11/09
MG# 28567
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

NDC 0095-0202-15
15 mL
VōSoL®
Otic Solution
(Acetic acid otic solution, USP)
VōSoL is a nonaqueous solution containing acetic acid (2%) in a propylene glycol vehicle containing propylene glycol diacetate (3%), benzethonium chloride (0.02%), and sodium acetate (0.015%).
Rx Only
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0095-0202-15
15 mL
VōSoL®
Otic Solution
(Acetic acid otic solution, USP)
Rx Only
VoSoLacetic acid SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||