Zinecard
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZINECARD safely and effectively. See full prescribing information for ZINECARD. ZINECARD (dexrazoxane) for injectionInitial U.S. Approval: 1995INDICATIONS AND USAGEZINECARD is a cytoprotective agent indicated for reducing the incidence and severity of cardiomyopathy associated with doxorubicin administration in women with metastatic breast cancer who have received a cumulative doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin therapy to maintain tumor control. Do not use ZINECARD with doxorubicin initiation. (1)DOSAGE AND ADMINISTRATION Reconstitute vial contents and dilute before use. (2.3) Administer ZINECARD by intravenous infusion over 15 minutes. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH. (2.1, 2.3) The recommended dosage ratio of ZINECARD to doxorubicin is 10:1 (e.g., 500 mg/m2 ZINECARD to 50 mg/m2 doxorubicin). Do not administer doxorubicin before ZINECARD. (2.1) Reduce dose by 50% for patients with creatinine clearance
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ZINECARD INDICATIONS AND USAGE
- 2 ZINECARD DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ZINECARD CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ZINECARD ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ZINECARD DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL - 250 mg Single-Dose Vial Label
- PRINCIPAL DISPLAY PANEL - 250 mg Single-Dose Vial Carton
- PRINCIPAL DISPLAY PANEL - 500 mg Single-Dose Vial Label
- PRINCIPAL DISPLAY PANEL - 500 mg Single-Dose Vial Carton
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ZINECARD is indicated for reducing the incidence and severity of cardiomyopathy associated with doxorubicin administration in women with metastatic breast cancer who have received a cumulative doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin therapy to maintain tumor control. Do not use with the initiation of doxorubicin therapy [see Warnings and Precautions (5.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
Administer ZINECARD Injection via intravenous infusion over 15 minutes. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH.
The recommended dosage ratio of ZINECARD to doxorubicin is 10:1 (e.g., 500 mg/m2 ZINECARD to 50 mg/m2 doxorubicin). Do not administer doxorubicin before ZINECARD. Administer doxorubicin within 30 minutes after the completion of ZINECARD infusion.
2.2 Dose Modifications
Dosing in Patients with Renal Impairment
Reduce ZINECARD dosage in patients with moderate to severe renal impairment (creatinine clearance values less than 40 mL/min) by 50% (ZINECARD to doxorubicin ratio reduced to 5:1; such as 250 mg/m2 ZINECARD to 50 mg/m2 doxorubicin) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Dosing in Patients with Hepatic Impairment
Since a doxorubicin dose reduction is recommended in the presence of hyperbilirubinemia, reduce the ZINECARD dosage proportionately (maintaining the 10:1 ratio) in patients with hepatic impairment.
2.3 Preparation and Administration
Preparation and Handling of Infusion Solution
Reconstitute ZINECARD with Sterile Water for Injection, USP. Reconstitute with 25 mL for a ZINECARD 250 mg vial and 50 mL for a ZINECARD 500 mg vial to give a concentration of 10 mg/mL. Dilute the reconstituted solution further with Lactated Ringer's Injection, USP to a concentration of 1.3 to 3.0 mg/mL in intravenous infusion bags for intravenous infusion.
Following reconstitution with Sterile Water for Injection, USP, ZINECARD is stable for 30 minutes at room temperature or if storage is necessary, up to 3 hours from the time of reconstitution when stored under refrigeration, 2° to 8°C (36° to 46°F). The pH of the resultant solution is 1.0 to 3.0. DISCARD UNUSED SOLUTIONS. The diluted infusion solutions are stable for one hour at room temperature or if storage is necessary, up to 4 hours when stored under refrigeration, 2° to 8°C (36° to 46°F). The infusion solutions have a pH of 3.5 to 5.5. DISCARD UNUSED SOLUTIONS.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Solutions containing a precipitate should be discarded.
Use caution when handling and preparing the reconstituted solution. The use of gloves is recommended. If ZINECARD powder or solutions contact the skin or mucosae, wash exposed area immediately and thoroughly with soap and water. Follow special handling and disposal procedures.1
Administration
Do not mix ZINECARD with other drugs.
Administer the final diluted solution of ZINECARD by intravenous infusion over 15 minutes before the administration of doxorubicin. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH. Administer doxorubicin within 30 minutes after the completion of ZINECARD infusion.
3 DOSAGE FORMS AND STRENGTHS
ZINECARD (dexrazoxane for injection) is available in 250 mg or 500 mg single dose vials as sterile, pyrogen-free lyophilizates.
4 CONTRAINDICATIONS
Do not use ZINECARD with non-anthracycline chemotherapy regimens.
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
ZINECARD may add to the myelosuppression caused by chemotherapeutic agents. Obtain a complete blood count prior to and during each course of therapy, and administer ZINECARD and chemotherapy only when adequate hematologic parameters are met.
5.2 Concomitant Chemotherapy
Only use ZINECARD in those patients who have received a cumulative doxorubicin dose of 300 mg/m2 and are continuing with doxorubicin therapy. Do not use with chemotherapy initiation as ZINECARD may interfere with the antitumor activity of the chemotherapy regimen. In a trial conducted in patients with metastatic breast cancer who were treated with fluorouracil, doxorubicin, and cyclophosphamide (FAC) with or without ZINECARD starting with their first cycle of FAC therapy, patients who were randomized to receive ZINECARD had a lower response rate (48% vs. 63%) and shorter time to progression than patients who were randomized to receive placebo.
5.3 Cardiac Toxicity
Treatment with ZINECARD does not completely eliminate the risk of anthracycline-induced cardiac toxicity. Monitor cardiac function before and periodically during therapy to assess left ventricular ejection fraction (LVEF). In general, if test results indicate deterioration in cardiac function associated with doxorubicin, the benefit of continued therapy should be carefully evaluated against the risk of producing irreversible cardiac damage.
5.4 Secondary Malignancies
Secondary malignancies such as acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) have been reported in studies of pediatric patients who have received ZINECARD in combination with chemotherapy. ZINECARD is not indicated for use in pediatric patients. Some adult patients who received ZINECARD in combination with anti-cancer agents known to be carcinogenic have also developed secondary malignancies, including AML and MDS.
Razoxane is the racemic mixture, of which dexrazoxane is the S(+)-enantiomer. Secondary malignancies (primarily acute myeloid leukemia) have been reported in patients treated chronically with oral razoxane. In these patients, the total cumulative dose of razoxane ranged from 26 to 480 grams and the duration of treatment was from 42 to 319 weeks. One case of T-cell lymphoma, one case of B-cell lymphoma, and six to eight cases of cutaneous basal cell or squamous cell carcinoma have also been reported in patients treated with razoxane. Long-term administration of razoxane to rodents was associated with the development of malignancies [see Nonclinical Toxicology (13.1)].
5.5 Embryo-Fetal Toxicity
ZINECARD can cause fetal harm when administered to pregnant women. Dexrazoxane administration during the period of organogenesis resulted in maternal toxicity, embryotoxicity and teratogenicity in rats and rabbits at doses significantly lower than the clinically recommended dose [see Use in Specific Populations (8.1)]. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Advise female patients of reproductive potential to avoid becoming pregnant and to use highly effective contraception during treatment [see Use in Specific Populations (8.6)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
The adverse reaction profile described in this section was identified from randomized, placebo-controlled, double-blind studies in patients with metastatic breast cancer who received the combination of the FAC chemotherapy regimen with or without ZINECARD. The dose of doxorubicin was 50 mg/m2 in each of these trials. Treatment was administered every three weeks until disease progression or cardiac toxicity.
Patients in clinical trials who received FAC with ZINECARD experienced more severe leukopenia, granulocytopenia, and thrombocytopenia than patients receiving FAC without ZINECARD [see Warnings and Precautions (5.1)].
Table 1 below lists the incidence of adverse reactions for patients receiving FAC with either ZINECARD or placebo in the breast cancer studies. Adverse experiences occurring during courses 1 through 6 are displayed for patients receiving ZINECARD or placebo with FAC beginning with their first course of therapy (columns 1 and 3, respectively). Adverse experiences occurring at course 7 and beyond for patients who received placebo with FAC during the first six courses and who then received either ZINECARD or placebo with FAC are also displayed (columns 2 and 4, respectively).
The adverse reactions listed below in Table 1 demonstrate that the frequency of adverse reaction "Pain on Injection" has been greater for ZINECARD arm, as compared to placebo.
| Adverse Reaction | Percentage (%) of Breast Cancer Patients With Adverse Reaction | |||
|---|---|---|---|---|
| FAC + ZINECARD | FAC + Placebo | |||
| Courses 1–6 N = 413 |
Courses ≥ 7 N = 102 |
Courses 1–6 N = 458 |
Courses ≥ 7 N = 99 |
|
| Alopecia | 94 | 100 | 97 | 98 |
| Nausea | 77 | 51 | 84 | 60 |
| Vomiting | 59 | 42 | 72 | 49 |
| Fatigue/Malaise | 61 | 48 | 58 | 55 |
| Anorexia | 42 | 27 | 47 | 38 |
| Stomatitis | 34 | 26 | 41 | 28 |
| Fever | 34 | 22 | 29 | 18 |
| Infection | 23 | 19 | 18 | 21 |
| Diarrhea | 21 | 14 | 24 | 7 |
| Pain on Injection | 12 | 13 | 3 | 0 |
| Sepsis | 17 | 12 | 14 | 9 |
| Neurotoxicity | 17 | 10 | 13 | 5 |
| Streaking/Erythema | 5 | 4 | 4 | 2 |
| Phlebitis | 6 | 3 | 3 | 5 |
| Esophagitis | 6 | 3 | 7 | 4 |
| Dysphagia | 8 | 0 | 10 | 5 |
| Hemorrhage | 2 | 3 | 2 | 1 |
| Extravasation | 1 | 3 | 1 | 2 |
| Urticaria | 2 | 2 | 2 | 0 |
| Recall Skin Reaction | 1 | 1 | 2 | 0 |
7 DRUG INTERACTIONS
No drug interactions have been identified [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D
Risk Summary
ZINECARD can cause fetal harm when administered to pregnant women. Dexrazoxane administration resulted in maternal toxicity, embryotoxicity and teratogenicity in rats and rabbits at doses significantly lower than the clinically recommended dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Warnings and Precautions (5.5)].
Animal Data
Dexrazoxane resulted in maternal toxicity in rats at doses of ≥2 mg/kg (1/40 the human dose on a mg/m2 basis) and embryotoxicity and teratogenicity at 8 mg/kg (approximately 1/10 the human dose on a mg/m2 basis) when given daily to pregnant rats during the period of organogenesis. Teratogenic effects in the rat included imperforate anus, microphthalmia, and anophthalmia. In offspring allowed to develop to maturity, fertility was impaired in the male and female rats treated in utero during organogenesis at 8 mg/kg. In rabbits, doses of ≥5 mg/kg (approximately 1/10 the human dose on a mg/m2 basis) daily during the period of organogenesis caused maternal toxicity and doses of 20 mg/kg (1/2 the human dose on a mg/m2 basis) were embryotoxic and teratogenic. Teratogenic effects in the rabbit included several skeletal malformations such as short tail, rib and thoracic malformations, and soft tissue variations including subcutaneous, eye and cardiac hemorrhagic areas, as well as agenesis of the gallbladder and of the intermediate lobe of the lung.
8.3 Nursing Mothers
It is not known whether dexrazoxane or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from dexrazoxane, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of dexrazoxane in pediatric patients have not been established [see Warnings and Precautions (5.4)].
8.5 Geriatric Use
Clinical studies of ZINECARD did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Females of Reproductive Potential
Contraception
ZINECARD can cause fetal harm when administered during pregnancy. Advise female patients of reproductive potential to use highly effective contraception during treatment [see Use in Specific Populations (8.1)].
8.7 Renal Impairment
Greater exposure to dexrazoxane may occur in patients with compromised renal function. Reduce the ZINECARD dose by 50% in patients with creatinine clearance values <40 mL/min [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There are no data on overdosage in the cardioprotective trials; the maximum dose administered during the cardioprotective trials was 1000 mg/m2 every three weeks.
Disposition studies with ZINECARD have not been conducted in cancer patients undergoing dialysis, but retention of a significant dose fraction (>0.4) of the unchanged drug in the plasma pool, minimal tissue partitioning or binding, and availability of greater than 90% of the systemic drug levels in the unbound form suggest that it could be removed using conventional peritoneal or hemodialysis.
There is no known antidote for dexrazoxane. Instances of suspected overdose should be managed with good supportive care until resolution of myelosuppression and related conditions is complete. Management of overdose should include treatment of infections, fluid regulation, and maintenance of nutritional requirements.
11 DESCRIPTION
ZINECARD (dexrazoxane for injection), a cardioprotective agent for use in conjunction with doxorubicin, is a sterile, pyrogen-free lyophilizate intended for intravenous administration.
Chemically, dexrazoxane is (S)-4,4'-(1-methyl-1,2-ethanediyl)bis-2,6-piperazinedione. The structural formula is as follows:
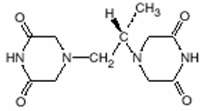
Dexrazoxane, an intracellular chelating agent, is a derivative of EDTA. Dexrazoxane is a whitish crystalline powder that melts at 191° to 197°C. It is sparingly soluble in water and 0.1 N HCl, slightly soluble in ethanol and methanol, and practically insoluble in nonpolar organic solvents. The pKa is 2.1. Dexrazoxane has an octanol/water partition coefficient of 0.025 and degrades rapidly above a pH of 7.0.
Each 250 mg vial contains dexrazoxane hydrochloride equivalent to 250 mg dexrazoxane. Hydrochloric Acid, NF is added for pH adjustment. When reconstituted as directed with 25 mL of Sterile Water for Injection, USP, each mL contains: 10 mg dexrazoxane. The pH of the resultant solution is 1.0 to 3.0.
Each 500 mg vial contains dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane. Hydrochloric Acid, NF is added for pH adjustment. When reconstituted as directed with 50 mL of Sterile Water for Injection, USP, each mL contains: 10 mg dexrazoxane. The pH of the resultant solution is 1.0 to 3.0.
The reconstituted ZINECARD solutions prepared from Sterile Water for Injection, USP, are intended for further dilution with Lactated Ringer's Injection, USP, for rapid intravenous drip infusion. DO NOT ADMINISTER VIA AN INTRAVENOUS PUSH [see Dosage and Administration (2.1, 2.3)].
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which ZINECARD exerts its cytoprotective activity is not fully understood. Dexrazoxane is a cyclic derivative of EDTA that penetrates cell membranes. Results of laboratory studies suggest that dexrazoxane is converted intracellularly to a ring-opened chelating agent that interferes with iron-mediated free radical generation thought to be responsible, in part, for anthracycline-induced cardiomyopathy.
12.3 Pharmacokinetics
The pharmacokinetics of dexrazoxane have been studied in advanced cancer patients with normal renal and hepatic function. The pharmacokinetics of dexrazoxane can be adequately described by a two-compartment open model with first-order elimination. Dexrazoxane has been administered as a 15 minute infusion over a dose range of 60 to 900 mg/m2 with 60 mg/m2 of doxorubicin, and at a fixed dose of 500 mg/m2 with 50 mg/m2 doxorubicin. The disposition kinetics of dexrazoxane are dose-independent, as shown by linear relationship between the area under plasma concentration-time curves and administered doses ranging from 60 to 900 mg/m2. The mean peak plasma concentration of dexrazoxane was 36.5 µg/mL at 15- minute after intravenous administration of 500 mg/m2 dose of ZINECARD over 15 to 30 minutes prior to the 50 mg/m2 doxorubicin dose.
The important pharmacokinetic parameters of dexrazoxane are summarized in Table 2:
| Dose Doxorubicin (mg/m2) | Dose ZINECARD (mg/m2) | Number of Subjects | Elimination Half-Life (h) | Plasma Clearance (L/h/m2) | Renal Clearance (L/h/m2) |
|
|---|---|---|---|---|---|---|
| 50 | 500 | 10 | 2.5 (16) | 7.88 (18) | 3.35 (36) | 22.4 (22) |
| 60 | 600 | 5 | 2.1 (29) | 6.25 (31) | — | 22.0 (55) |
Distribution
Following a rapid distributive phase (0.2 to 0.3 hours), dexrazoxane reaches post-distributive equilibrium within two to four hours. The estimated mean steady-state volume of distribution of dexrazoxane is 22.4 L/m2 after 500 mg/m2 of ZINECARD dose followed by 50 mg/m2 of doxorubicin, suggesting distribution throughout total body water (25 L/m2).
In vitro studies have shown that dexrazoxane is not bound to plasma proteins.
Metabolism
Qualitative metabolism studies with dexrazoxane have confirmed the presence of unchanged drug, a diacid-diamide cleavage product, and two monoacid-monoamide ring products in the urine of animals and man. The metabolite levels were not measured in the pharmacokinetic studies.
Excretion
Urinary excretion plays an important role in the elimination of dexrazoxane. Forty-two percent of a 500 mg/m2 dose of ZINECARD was excreted in the urine. Renal clearance averages 3.35 L/h/m2 after the 500 mg/m2 ZINECARD dose followed by 50 mg/m2 of doxorubicin.
Specific Populations
Pediatric
Pharmacokinetics following ZINECARD administration have not been evaluated in pediatric patients.
Effect of Renal Impairment
The pharmacokinetics of dexrazoxane were assessed following a single 15-minute IV infusion of 150 mg/m2 of ZINECARD. Dexrazoxane clearance was reduced in subjects with renal dysfunction. Compared with controls, the mean AUC0–inf value was two-fold greater in subjects with moderate (CLCR 30–50 mL/min) to severe (CLCR <30 mL/min) renal dysfunction. Modeling demonstrated that equivalent exposure (AUC-inf) could be achieved if dosing were reduced by 50% in subjects with creatinine clearance values <40 mL/min compared with control subjects (CLCR >80 mL/min) [see Use in Specific Populations (8.7) and Dosage and Administration (2.2)].
Effect of Hepatic Impairment
Pharmacokinetics following ZINECARD administration have not been evaluated in patients with hepatic impairment. The ZINECARD dose is dependent upon the dose of doxorubicin [see Dosage and Administration (2.2)].
Drug Interactions
There was no significant change in the pharmacokinetics of doxorubicin (50 mg/m2) and its predominant metabolite, doxorubicinol, in the presence of dexrazoxane (500 mg/m2) in a crossover study in cancer patients.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term carcinogenicity studies have been carried out with dexrazoxane in animals. Nevertheless, a study by the National Cancer Institute has reported that long-term dosing with razoxane (the racemic mixture of dexrazoxane, ICRF-187, and its enantiomer ICRF-186) is associated with the development of malignancies in rats and possibly in mice [see Warnings and Precautions (5.4)].
Dexrazoxane was not mutagenic in the bacterial reverse mutation (Ames) test, but was found to be clastogenic to human lymphocytes in vitro and to mouse bone marrow erythrocytes in vivo (micronucleus test).
ZINECARD has the potential to impair fertility in male patients based on effects in repeat-dose toxicology studies. Testicular atrophy was seen with dexrazoxane administration at doses as low as 30 mg/kg weekly for 6 weeks in rats (1/3 the human dose on a mg/m2 basis) and as low as 20 mg/kg weekly for 13 weeks in dogs (approximately equal to the human dose on a mg/m2 basis).
14 CLINICAL STUDIES
The ability of ZINECARD to prevent/reduce the incidence and severity of doxorubicin-induced cardiomyopathy was evaluated in three prospectively randomized placebo-controlled studies. In these studies, patients were treated with a doxorubicin-containing regimen and either ZINECARD or placebo starting with the first course of chemotherapy. There was no restriction on the cumulative dose of doxorubicin. Cardiac function was assessed by measurement of the LVEF, utilizing resting multigated nuclear medicine (MUGA) scans, and by clinical evaluations. Patients receiving ZINECARD had significantly smaller mean decreases from baseline in LVEF and lower incidences of congestive heart failure than the control group; however, in the largest study, patients with advanced breast cancer receiving FAC with ZINECARD had a lower response rate (48% vs. 63%) and a shorter time to progression than patients who received FAC versus placebo.
In the clinical trials, patients who were initially randomized to receive placebo were allowed to receive ZINECARD after a cumulative dose of doxorubicin above 300 mg/m2. Retrospective historical analyses showed that the risk of experiencing a cardiac event (see Table 3 for definition) at a cumulative dose of doxorubicin above 300 mg/m2 was greater in the patients who did not receive ZINECARD beginning with their seventh course of FAC than in the patients who did receive ZINECARD (HR=13.08; 95% CI: 3.72, 46.03; p<0.001). Overall, 3% of patients treated with ZINECARD developed CHF compared with 22% of patients not receiving ZINECARD.
|
Figure 1 shows the number of patients still on treatment at increasing cumulative doses.
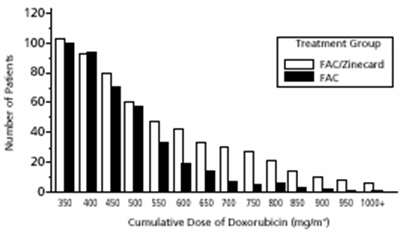
|
15 REFERENCES
- "OSHA Hazardous Drugs." OSHA http://www.osha.gov/SLTC/hazardousdrugs/index.html.
16 HOW SUPPLIED/STORAGE AND HANDLING
ZINECARD (dexrazoxane for injection) is available in the following strengths as sterile, pyrogen-free lyophilizates.
NDC 0013-8717-62
250 mg single dose vial with a red flip-top seal, packaged in single vial packs.
NDC 0013-8727-89
500 mg single dose vial with a blue flip-top seal, packaged in single vial packs.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Follow special handling and disposal procedures.1
17 PATIENT COUNSELING INFORMATION
17.1 Myelosuppression
Treatment with ZINECARD is associated with leukopenia, neutropenia, and thrombocytopenia. Perform hematological monitoring [see Warnings and Precautions (5.1), (5.6)].
17.2 Embryo-Fetal Toxicity
Counsel patients on pregnancy planning and prevention. Advise female patients of reproductive potential that ZINECARD can cause fetal harm and to use highly effective contraception during treatment [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1, 8.6)].
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.

LAB-0060-9.0
PRINCIPAL DISPLAY PANEL - 250 mg Single-Dose Vial Label
NDC 0013-8717-62
Single-Dose Vial
Zinecard
®
(dexrazoxane) for
injection
250 mg*
Sterile, Pyrogen-Free
Lyophilizate
For Intravenous Use Only
Rx only
PRINCIPAL DISPLAY PANEL - 250 mg Single-Dose Vial Carton
NDC 0013-8717-62
Single-Dose Vial
Zinecard
®
(dexrazoxane) for
injection
250 mg*
Sterile, Pyrogen-Free
Lyophilizate
For Intravenous Use Only
Pfizer Injectables
Rx only
PRINCIPAL DISPLAY PANEL - 500 mg Single-Dose Vial Label
NDC 0013-8727-89
Single-Dose Vial
Zinecard
®
(dexrazoxane) for injection
500 mg*
Sterile, Pyrogen-Free
Lyophilizate
For Intravenous Use Only
Rx only
PRINCIPAL DISPLAY PANEL - 500 mg Single-Dose Vial Carton
NDC 0013-8727-89
Single-Dose Vial
Zinecard
®
(dexrazoxane) for
injection
500 mg*
Sterile, Pyrogen-Free
Lyophilizate
For Intravenous Use Only
Pfizer Injectables
Rx only

Zinecarddexrazoxane INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zinecarddexrazoxane INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||