ZIRGAN
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZIRGAN safely and effectively. See full prescribing information for ZIRGAN.ZIRGAN (ganciclovir ophthalmic gel) 0.15%Initial U.S. Approval: 1989INDICATIONS AND USAGEZIRGAN is a topical ophthalmic antiviral that is indicated for the treatment of acute herpetic keratitis (dendritic ulcers). ( 1) DOSAGE AND ADMINISTRATIONThe recommended dosing regimen for ZIRGAN is 1 drop in the affected eye 5 times per day (approximately every 3 hours while awake) until the corneal ulcer heals, and then 1 drop 3 times per day for 7 days. (2) DOSAGE FORMS AND STRENGTHSZIRGAN contains 0.15% of ganciclovir in a sterile preserved topical ophthalmic gel. (3) CONTRAINDICATIONSNone. WARNINGS AND PRECAUTIONS ZIRGAN is indicated for topical ophthalmic use only. ( 5.1) Patients should not wear contact lenses if they have signs or symptoms of herpetic keratitis or during the course of therapy with ZIRGAN. (5.2) Side EffectsMost common adverse reactions reported in patients were blurred vision (60%), eye irritation (20%), punctate keratitis (5%), and conjunctival hyperemia (5%). ( 6) To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb at 1-800-323-0000 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ZIRGAN INDICATIONS AND USAGE
- 2 ZIRGAN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ZIRGAN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ZIRGAN ADVERSE REACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 11 ZIRGAN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ZIRGAN (ganciclovir ophthalmic gel) 0.15% is indicated for the treatment of acute herpetic keratitis (dendritic ulcers).
2 DOSAGE AND ADMINISTRATION
The recommended dosing regimen for ZIRGAN is 1 drop in the affected eye 5 times per day (approximately every 3 hours while awake) until the corneal ulcer heals, and then 1 drop 3 times per day for 7 days.
3 DOSAGE FORMS AND STRENGTHS
ZIRGAN contains 0.15% of ganciclovir in a sterile preserved topical ophthalmic gel.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
ZIRGAN is indicated for topical ophthalmic use only.
5.2 Avoidance of Contact Lenses
Patients should not wear contact lenses if they have signs or symptoms of herpetic keratitis or during the course of therapy with ZIRGAN.
6 ADVERSE REACTIONS
Most common adverse reactions reported in patients were blurred vision (60%), eye irritation (20%), punctate keratitis (5%), and conjunctival hyperemia (5%).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Teratogenic Effects
Pregnancy Category C. Ganciclovir has been shown to be embryotoxic in rabbits and mice following intravenous administration and teratogenic in rabbits. Fetal resorptions were present in at least 85% of rabbits and mice administered 60 mg/kg/day and 108 mg/kg/day (approximately 10,000x and 17,000x the human ocular dose of 6.25 mcg/kg/day), respectively, assuming complete absorption. Effects observed in rabbits included: fetal growth retardation, embryolethality, teratogenicity, and/or maternal toxicity. Teratogenic changes included cleft palate, anophthalmia/microphthalmia, aplastic organs (kidney and pancreas), hydrocephaly, and brachygnathia. In mice, effects observed were maternal/fetal toxicity and embryolethality. Daily intravenous doses of 90 mg/kg/day (14,000x the human ocular dose) administered to female mice prior to mating, during gestation, and during lactation caused hypoplasia of the testes and seminal vesicles in the month-old male offspring, as well as pathologic changes in the nonglandular region of the stomach (see Carcinogenesis, Mutagenesis, and Impairment of Fertility).
There are no adequate and well-controlled studies in pregnant women. ZIRGAN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether topical ophthalmic ganciclovir administration could result in sufficient systemic absorption to produce detectable quantities in breast milk. Caution should be exercised when ZIRGAN is administered to nursing mothers.
8.4 Pediatric Use
Safety and efficacy in pediatric patients below the age of 2 years have not been established.
8.5 Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
11 DESCRIPTION
ZIRGAN (ganciclovir ophthalmic gel) 0.15% contains a sterile, topical antiviral for ophthalmic use. The chemical name is 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine (CAS number 82410-32-0). Ganciclovir is represented by the following structural formula:
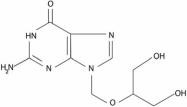
Ganciclovir has a molecular weight of 255.23, and the empirical formula is C9H13N5O4.
Each gram of gel contains: ACTIVE: ganciclovir 1.5 mg (0.15%). INACTIVES: carbopol, water for injection, sodium hydroxide (to adjust the pH to 7.4), mannitol. PRESERVATIVE: benzalkonium chloride 0.075 mg.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ZIRGAN (ganciclovir ophthalmic gel) 0.15% contains the active ingredient, ganciclovir, which is a guanosine derivative that, upon phosphorylation, inhibits DNA replication by herpes simplex viruses (HSV). Ganciclovir is transformed by viral and cellular thymidine kinases (TK) to ganciclovir triphosphate, which works as an antiviral agent by inhibiting the synthesis of viral DNA in 2 ways: competitive inhibition of viral DNA-polymerase and direct incorporation into viral primer strand DNA, resulting in DNA chain termination and prevention of replication.
12.3 Pharmacokinetics
The estimated maximum daily dose of ganciclovir administered as 1 drop, 5 times per day is 0.375 mg. Compared to maintenance doses of systemically administered ganciclovir of 900 mg (oral valganciclovir) and 5 mg/kg (IV ganciclovir), the ophthalmically administered daily dose is approximately 0.04% and 0.1% of the oral dose and IV doses, respectively, thus minimal systemic exposure is expected.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ganciclovir was carcinogenic in the mouse at oral doses of 20 and 1,000 mg/kg/day (approximately 3,000x and 160,000x the human ocular dose of 6.25 mcg/kg/day, assuming complete absorption). At the dose of 1,000 mg/kg/day there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland, and vagina) and liver in females. At the dose of 20 mg/kg/day, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. No carcinogenic effect was observed in mice administered ganciclovir at 1 mg/kg/day (160x the human ocular dose). Except for histocytic sarcoma of the liver, ganciclovir-induced tumors were generally of epithelial or vascular origin. Although the preputial and clitoral glands, forestomach and harderian glands of mice do not have human counterparts, ganciclovir should be considered a potential carcinogen in humans. Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro at concentrations between 50 to 500 and 250 to 2,000 mcg/mL, respectively.
In the mouse micronucleus assay, ganciclovir was clastogenic at doses of 150 and 500 mg/kg (IV) (24,000x to 80,000x human ocular dose) but not 50 mg/kg (8,000x human ocular dose). Ganciclovir was not mutagenic in the Ames Salmonella assay at concentrations of 500 to 5,000 mcg/mL.
Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following intravenous doses of 90 mg/kg/day (approximately 14,000x the human ocular dose of 6.25 mcg/kg/day). Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration of doses ranging from 0.2 to 10 mg/kg (30x to 1,600x the human ocular dose).
14 CLINICAL STUDIES
In one open-label, randomized, controlled, multicenter clinical trial which enrolled 164 patients with herpetic keratitis, ZIRGAN was non-inferior to acyclovir ophthalmic ointment, 3% in patients with dendritic ulcers. Clinical resolution (healed ulcers) at Day 7 was achieved in 77% (55/71) for ZIRGAN versus 72% (48/67) for acyclovir 3% (difference 5.8%, 95% CI -9.6%-18.3%). In three randomized, single-masked, controlled, multicenter clinical trials which enrolled 213 total patients, ZIRGAN was non‑inferior to acyclovir ophthalmic ointment 3% in patients with dendritic ulcers. Clinical resolution at Day 7 was achieved in 72% (41/57) for ZIRGAN versus 69% (34/49) for acyclovir (difference 2.5%, 95% CI -15.6%-20.9%).
16 HOW SUPPLIED/STORAGE AND HANDLING
ZIRGAN is supplied as 5 grams of a sterile, preserved, clear, colorless, topical ophthalmic gel containing O.15% of ganciclovir in a polycoated aluminum tube with a white polyethylene tip and cap and protective band (NDC 24208-535-35).
Storage
Store at 15°C-25°C (59°F-77°F). Do not freeze.
17 PATIENT COUNSELING INFORMATION
This product is sterile when packaged. Patients should be advised not to allow the dropper tip to touch any surface, as this may contaminate the gel. If pain develops, or if redness, itching, or inflammation becomes aggravated, the patient should be advised to consult a physician. Patients should be advised not to wear contact lenses when using ZIRGAN.
Revised: June 2010
ZIRGAN is a trademark of Laboratoires Théa Corporation licensed by Bausch & Lomb Incorporated.
Bausch & Lomb Incorporated
Tampa, FL 33637
© Bausch & Lomb Incorporated
9187201
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL

NDC 24208-535-35
Zirgan®
ganciclovir ophthalmic gel
0.15%
Rx only
5 gm
Sterile
Bausch & Lomb
ZIRGANganciclovir GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||