ZOMIG
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZOMIG NASAL SPRAY. See full prescribing information for ZOMIG (zolmitriptan) NASAL SPRAYINITIAL U.S. APPROVAL: 1997RECENT MAJOR CHANGESWarning and Precautions, serotonin syndrome (5.5) 10/2008 Drug Interactions, serotonin syndrome (7.5) 10/2008 Use in Specific Populations, pediatric use (8.4) 10/2008INDICATIONS AND USAGEZOMIG Nasal Spray is a 5HT1B/1D receptor agonist (triptan) indicated for: Acute treatment of migraine with or without aura in adults (1) Important limitations: Use only after a clear diagnosis of migraine has been established (1.2) Not intended for the prophylactic therapy of migraine (1.2) Not indicated for the treatment of cluster headache (1.2) DOSAGE AND ADMINISTRATIONSingle 5 mg dose; may repeat after 2 hours if needed; not to exceed 10 mg in any 24-hour period; benefit of a second dose not established (2)DOSAGE FORMS AND STRENGTHSNasal Spray: 5 mg (3)CONTRAINDICATIONS Ischemic heart disease, coronary artery vasospasm, or other significant underlying cardiovascular disease (4.1) Cerebrovascular syndromes (e.g. history of stroke or TIA) (4.2) Peripheral Vascular Disease (including ischemic bowel disease) (4.3) Uncontrolled hypertension (4.4) Do not use ZOMIG within 24 hours of another 5-HT1 agonist, ergotamine-containing or ergot-type medication (4.5) Hemiplegic or basilar migraine (4.6) Do not use ZOMIG within 2 weeks of an MAO-A inhibitor (4.7) Hypersensitivity to ZOMIG (4.8) WARNINGS AND PRECAUTIONS Serious adverse cardiac events, including acute myocardial infarction, and life-threatening disturbances of cardiac rhythm (5.1) It is strongly recommended that ZOMIG not be given to patients in whom unrecognized coronary artery disease (CAD) is predicted by the presence of risk factors. In very rare cases, serious cardiovascular events have been reported in association with ZOMIG in the absence of known cardiovascular disease. If ZOMIG is considered, patients should first have a cardiovascular evaluation. If the evaluation is satisfactory, first dose should take place in a physician’s office setting (5.1) Sensations of pain, tightness, pressure and heaviness in the chest, throat, neck and jaw: generally not associated with myocardial ischemia, but patients with signs or symptoms suggestive of angina should be evaluated for the presence of CAD (5.2) Cerebrovascular events, some fatal (5.3) Gastrointestinal ischemic events and peripheral vasospastic reactions (e.g. Raynaud’s syndrome) (5.4) Patients with symptomatic Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathways should not receive ZOMIG (5.1) Potentially life-threatening serotonin syndrome, particularly in combination with SSRIs or SNRIs. Monitor patients carefully if concomitant treatment is clinically warranted (5.5, 7.6) Increase in blood pressure, very rarely associated with significant clinical events (4.4, 5.6) Side Effects In controlled studies the most common adverse reactions (≥ 2% and > placebo) were: unusual taste, paresthesia, hyperesthesia, nausea, pain location specified, pain throat, somnolence, asthenia, disorder/discomfort of nasal cavity, dry mouth, tightness throat (6.1) To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Ergot-type or ergotamine-containing medications other 5HT1 agonists, and ZOMIG: do not use within 24 hours of each other (4.5, 7.1, 7.3) Do not use ZOMIG within 2 weeks of an MAO-A inhibitor (4.7, 7.2) Cimetidine: half-life and AUC of zolmitriptan doubled (7.4) SSRI or SNRI: life-threatening serotonin syndrome reported during combined use with triptans (5.5, 7.5) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm. Use ZOMIG during pregnancy only if the potential benefit justifies the potential risk to the fetus (8.1) Nursing Mothers: Use with caution while nursing, as it is not known if ZOMIG is excreted in human milk. Zolmitriptan has been detected in rat milk at levels equal to or greater than those in maternal plasma (8.3) Pediatric Use: Efficacy not established in a study in patients 12-17 years. Adverse reactions similar in nature and frequency to adults. Not studied in patients under 12 years (8.4) Geriatric Use: Safety and effectiveness in patients over 65 not established (8.5, 12.3) Hepatic Impairment: Decreased clearance of zolmitriptan and significant elevation in blood pressure observed. Use doses < 2.5 mg of an oral formulation, with blood pressure monitoring (2.2, 8.6, 12.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ZOMIG INDICATIONS AND USAGE
- 2 ZOMIG DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ZOMIG CONTRAINDICATIONS
- 4.1 Ischemic or Vasospastic Coronary Artery Disease
- 4.2 Cerebrovascular Syndromes
- 4.3 Peripheral Vascular Disease
- 4.4 Uncontrolled Hypertension
- 4.5 Use within 24 hours of treatment with another 5-HT agonist, or ergotamine containing medication, or ergot type medication
- 4.6 Hemiplegic or Basilar Migraine
- 4.7 Administration of MAO-A inhibitors within 2 weeks
- 4.8 Hypersensitivity to zolmitriptan
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Risk of Myocardial Ischemia and/or Infarction and Other Adverse Cardiac Events
- 5.2 Sensations of pain, tightness, pressure in the chest and or throat, neck and jaw
- 5.3 Cerebrovascular Events
- 5.4 Other Vasospasm-Related Events, including Peripheral Vascular Ischemia and Colonic Ischemia
- 5.5 Serotonin Syndrome
- 5.6 Increase in Blood Pressure
- 5.7 Binding to Melanin-Containing Tissues
- 5.8 Laboratory Tests
- 5.9 Drug/Laboratory Test Interactions
- 6 ZOMIG ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 ZOMIG DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.5 Approved Patient Labeling
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Acute Treatment of Migraine Attacks
ZOMIG Nasal Spray is indicated for the acute treatment of migraine with or without aura in adults.
1.2 Important Limitations
ZOMIG should only be used where a clear diagnosis of migraine has been established. If a patient has no response for the first migraine attack treated with ZOMIG, the diagnosis of migraine should be reconsidered before ZOMIG is administered to treat any subsequent attacks.
ZOMIG is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine [see Contraindications (4.6)].
Safety and effectiveness of ZOMIG have not been established for cluster headache, which is present in an older, predominantly male population.
2 DOSAGE AND ADMINISTRATION
2.1 Acute Treatment of Migraine Attacks
Administer one dose of ZOMIG Nasal Spray 5 mg for the treatment of acute migraine. If the headache returns, the dose may be repeated after 2 hours. The effectiveness of a second dose has not been established in placebo-controlled trials. The maximum daily dose should not exceed 10 mg in any 24-hour period.
In controlled clinical trials, single doses of 5 mg of zolmitriptan nasal spray were administered into one nostril and were effective for the treatment of acute migraines in adults.
Individuals may vary in response to ZOMIG Nasal Spray. The pharmacokinetics of a 5 mg nasal spray dose is similar to the 5 mg oral formulations. Doses lower than 5 mg can only be achieved through the use of an oral formulation. The choice of dose, and route of administration should therefore be made on an individual basis.
The safety of treating an average of more than four headaches in a 30-day period has not been established.
2.2 Hepatic Impairment
Patients with moderate to severe hepatic impairment have decreased clearance of zolmitriptan and significant elevation in blood pressure was observed in some patients. Use of doses less than 2.5mg of an alternate formulation with blood pressure monitoring is recommended [see Clinical Pharmacology (12.3) and Warnings and Precautions (5.6)].
3 DOSAGE FORMS AND STRENGTHS
Nasal Spray 5 mg
4 CONTRAINDICATIONS
4.1 Ischemic or Vasospastic Coronary Artery Disease
ZOMIG should not be given to patients with ischemic heart disease (angina pectoris, history of myocardial infarction, or documented silent ischemia) or to patients who have symptoms or findings consistent with ischemic heart disease, coronary artery vasospasm, including Prinzmetal’s variant angina, or other significant underlying cardiovascular disease [see Warnings and Precautions (5.1)].
4.2 Cerebrovascular Syndromes
ZOMIG should not be given to patients with cerebrovascular syndromes including (but not limited to) stroke of any type as well as transient ischemic attacks. [see Warnings and Precautions (5.3)].
4.3 Peripheral Vascular Disease
ZOMIG should not be given to patients with peripheral vascular disease including (but not limited to) ischemic bowel disease [see Warnings and Precautions (5.4)].
4.4 Uncontrolled Hypertension
Because ZOMIG may increase blood pressure, it should not be given to patients with uncontrolled hypertension [see Warnings and Precautions (5.6)].
4.5 Use within 24 hours of treatment with another 5-HT agonist, or ergotamine containing medication, or ergot type medication
ZOMIG and any ergotamine-containing or ergot-type medication (such as dihydroergotamine or methysergide) should not be used within 24 hours of each other, nor should ZOMIG and another 5-HT1 agonist be used within 24 hours of each other [See Drug Interactions (7.1 and 7.3)].
4.6 Hemiplegic or Basilar Migraine
ZOMIG should not be administered to patients with hemiplegic or basilar migraine.
4.7 Administration of MAO-A inhibitors within 2 weeks
Concurrent administration of MAO-A inhibitors or use of zolmitriptan within 2 weeks of discontinuation of MAO-A inhibitor therapy is contraindicated [see Clinical Pharmacology (12.4) and Drug Interactions (7.2)].
4.8 Hypersensitivity to zolmitriptan
ZOMIG is contraindicated in patients who are hypersensitive to zolmitriptan or any of its inactive ingredients.
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Myocardial Ischemia and/or Infarction and Other Adverse Cardiac Events
Cardiac Events and Fatalities with 5-HT1 Agonists
Serious adverse cardiac events, including acute myocardial infarction, have been reported within a few hours following administration of zolmitriptan. Life-threatening disturbances of cardiac rhythm, and death have been reported within a few hours following the administration of other 5-HT1 agonists. Considering the extent of use of 5-HT1 agonists in patients with migraine, the incidence of these events is extremely low.
ZOMIG can cause coronary artery vasospasm; at least one of these events occurred in a patient with no cardiac disease history and with documented absence of coronary artery disease. Because of the close proximity of the events to ZOMIG use, a causal relationship cannot be excluded. In the cases where there has been known underlying coronary artery disease, the relationship is uncertain. Patients who experience signs or symptoms suggestive of angina following dosing should be evaluated for the presence of CAD or a predisposition to Prinzmetal’s variant angina before receiving additional doses of medication, and should be monitored electrocardiographically if dosing is resumed and similar symptoms recur.
Patients with symptomatic Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders should not receive ZOMIG.
Premarketing experience with zolmitriptan
Among the more than 2,500 patients with migraine who participated in premarketing controlled clinical trials of ZOMIG Tablets, no deaths or serious cardiac events were reported. In a premarketing controlled clinical trial of ZOMIG Nasal Spray, more than 1,300 patients participated and there were no deaths or serious cardiac events to report.
Postmarketing experience with zolmitriptan
Serious cardiovascular events have been reported in association with the use of ZOMIG Tablets, and in very rare cases, these events have occurred in the absence of known cardiovascular disease. The uncontrolled nature of postmarketing surveillance, however, makes it impossible to determine definitively the proportion of the reported cases that were actually caused by zolmitriptan or to reliably assess causation in individual cases.
Patients with documented coronary artery disease
Because of the potential of this class of compound (5-HT1 agonists) to cause coronary vasospasm, ZOMIG should not be given to patients with documented ischemic or vasospastic coronary artery disease [see Contraindications (4.1)].
Patients with risk factors for CAD
It is strongly recommended that zolmitriptan not be given to patients in whom unrecognized coronary artery disease (CAD) is predicted by the presence of risk factors (eg, hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of CAD, female with surgical or physiological menopause, or male over 40 years of age) unless a cardiovascular evaluation provides satisfactory clinical evidence that the patient is reasonably free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. The sensitivity of cardiac diagnostic procedures to detect cardiovascular disease or predisposition to coronary artery vasospasm is modest, at best. If, during the cardiovascular evaluation, the patient’s medical history, electrocardiographic or other investigations reveal findings indicative of, or consistent with, coronary artery vasospasm or myocardial ischemia, zolmitriptan should not be administered [see Contraindications (4.1)].
For patients with risk factors predictive of CAD, who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of zolmitriptan take place in the setting of a physician’s office or similar medically staffed and equipped facility unless the patient has previously received zolmitriptan. Because cardiac ischemia can occur in the absence of clinical symptoms, consideration should be given to obtaining on the first occasion of use an electrocardiogram (ECG) during the interval immediately following ZOMIG, in these patients with risk factors.
It is recommended that patients who are intermittent long-term users of ZOMIG and who have or acquire risk factors predictive of CAD, as described above, undergo periodic interval cardiovascular evaluation as they continue to use ZOMIG.
The systematic approach described above is intended to reduce the likelihood that patients with unrecognized cardiovascular disease will be inadvertently exposed to zolmitriptan.
5.2 Sensations of pain, tightness, pressure in the chest and or throat, neck and jaw
As with other 5-HT1 agonists, sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw have been reported after treatment with ZOMIG Tablets.
Because 5-HT1 agonists may cause coronary vasospasm, patients who experience signs or symptoms suggestive of angina following dosing should be evaluated for the presence of CAD or a predisposition to Prinzmetal’s variant angina before receiving additional doses of medication, and should be monitored electrocardiographically if dosing is resumed and similar symptoms occur. Patients shown to have CAD and those with Prinzmetal’s variant angina should not receive 5-HT1 agonists [see Contraindications (4.1)].
5.3 Cerebrovascular Events
Cerebral hemorrhage, subarachnoid hemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, care should be taken to exclude other potentially serious neurological conditions. It should be noted that patients with migraine may be at increased risk of certain cerebrovascular events (eg, stroke, hemorrhage, transient ischemic attack) [see Contraindications (4.2)].
5.4 Other Vasospasm-Related Events, including Peripheral Vascular Ischemia and Colonic Ischemia
5-HT1 agonists, including ZOMIG, may cause vasospastic reactions other than coronary artery vasospasm, such as peripheral and gastrointestinal vascular ischemia with abdominal pain and bloody diarrhea.
Very rare reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Visual disorders may also be part of a migraine attack.
Patients who experience other symptoms or signs suggestive of decreased arterial flow following the use of any 5-HT agonist, such as ischemic bowel syndrome or Raynaud’s syndrome, are candidates for further evaluation [see Contraindications (4.3)].
5.5 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome may occur with triptans, including ZOMIG treatment, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs). If concomitant treatment with ZOMIG and an SSRI (e.g., fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, escitalopram) or SNRI (e.g., venlafaxine, duloxetine) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea) [See Drug Interactions (7.5)].
5.6 Increase in Blood Pressure
As with other 5-HT1 agonists, significant elevations in systemic blood pressure have been reported on rare occasions with ZOMIG Tablet use, in patients with and without a history of hypertension; very rarely these increases in blood pressure have been associated with significant clinical events. Zolmitriptan is contraindicated in patients with uncontrolled hypertension. In volunteers, an increase of 1 and 5 mm Hg in the systolic and diastolic blood pressure, respectively, was seen at 5 mg. In the headache trials, vital signs were measured only in the small inpatient study and no effect on blood pressure was seen. In a study of patients with moderate to severe liver disease, 7 of 27 experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a dose of 10 mg of zolmitriptan [see Contraindications (4.4)].
An 18% increase in mean pulmonary artery pressure was seen following dosing with another 5-HT1 agonist in a study evaluating subjects undergoing cardiac catheterization.
5.7 Binding to Melanin-Containing Tissues
When pigmented rats were given a single oral dose of 10 mg/kg of radiolabeled zolmitriptan, the radioactivity in the eye after 7 days, the latest time point examined, was still 75% of the value measured after 4 hours. This suggests that zolmitriptan and/or its metabolites may bind to the melanin of the eye. Because there could be accumulation in melanin rich tissues over time, this raises the possibility that zolmitriptan could cause toxicity in these tissues after extended use. However, no effects on the retina related to treatment with zolmitriptan were noted in any of the toxicity studies including those conducted by the nasal route. Although no systematic monitoring of ophthalmologic function was undertaken in clinical trials, and no specific recommendations for ophthalmologic monitoring are offered, prescribers should be aware of the possibility of long-term ophthalmologic effects.
5.8 Laboratory Tests
No monitoring of specific laboratory tests is recommended.
5.9 Drug/Laboratory Test Interactions
Zolmitriptan is not known to interfere with commonly employed clinical laboratory tests.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Serious cardiac reactions, including myocardial infarction, have occurred following the use of ZOMIG Tablets. These reactions are extremely rare and most have been reported in patients with risk factors predictive of CAD. Reactions reported, in association with triptans, have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation [see Contraindications (4.1) and Warnings and Precautions (5.1)].
Incidence in Controlled Clinical Trials:
Among 464 adult patients treating single attacks with zolmitriptan nasal spray in a blinded placebo controlled trial, there was a low withdrawal rate related to adverse reactions: 5 mg (1.3%), and placebo (0.4%). None of the withdrawals were due to a serious reaction. One patient was withdrawn due to abnormal ECG changes from baseline that was incidentally found 23 days after the last dose of ZOMIG Nasal Spray. The most common adverse reactions in clinical trials for ZOMIG Nasal Spray were: unusual taste, paresthesia, hyperesthesia, and dizziness.
Table 1 lists the adverse reactions that occurred in ≥ 2% of the 236 patients in the 5 mg dose group of the controlled clinical trial.
|
Body system and adverse reaction |
Placebo (N=228) |
5.0 mg (N=236) |
|
Atypical Sensations |
||
|
Hyperesthesia |
0% |
5% |
|
Paraesthesia |
6% |
10% |
|
Ear/Nose/Throat |
||
|
Disorder/Discomfort of nasal cavity |
2% |
3% |
|
Pain and Pressure Sensations |
||
|
Pain Location Specified |
1% |
4% |
|
Pain Throat |
1% |
4% |
|
Tightness Throat |
1% |
2% |
|
Digestive |
||
|
Dry Mouth |
0% |
2% |
|
Nausea |
1% |
4% |
|
Neurological |
||
|
Somnolence |
2% |
4% |
|
Unusual Taste |
3% |
21% |
|
Other |
||
|
Asthenia |
1% |
3% |
Adverse clinical reactions occurring in ≥ 1% and < 2% of patients in all attacks of the controlled clinical trial were pain abdominal, pressure throat, vomiting, headache, tightness chest, dysphagia, insomnia, palpitation and reaction aggravation.
The incidence of adverse reactions in controlled clinical trials was not affected by gender, weight, or age of the patients (18-39 vs. 40-65 years of age), or presence of aura. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Local Adverse Reactions:
Among 922 patients using the zolmitriptan nasal spray to treat 2311 attacks in the controlled clinical study who were exposed, across all doses (0.5 to 5 mg), approximately 3% noted local irritation or soreness at the site of administration. Adverse reactions of any kind, perceived in the nasopharynx (which may include systemic effects of triptans) were severe in about 1% of patients and approximately 60% resolved in 1 hour. Nasopharyngeal examinations, in a subset of patients participating in two long term trials of up to one year duration, failed to demonstrate any clinically significant changes with repeated use of ZOMIG Nasal Spray.
All nasopharyngeal adverse reactions with an incidence of ≥ 2% of patients in any zolmitriptan nasal spray dose groups are included in ADVERSE REACTIONS Table 1.
Other Adverse Reactions:
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. Because the reports include reactions observed in open and uncontrolled studies, the role of ZOMIG in their causation cannot be reliably determined. Furthermore, variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Reaction frequencies are calculated as the number of patients who used ZOMIG Nasal Spray and reported a reaction divided by the total number of patients exposed to ZOMIG Nasal Spray (n=3059). All reported reactions are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: infrequent adverse reactions are those occurring in 1/100 to 1/1,000 patients and rare adverse reactions are those occurring in fewer than 1/1,000 patients.
Body:
Infrequent: allergic reaction, back pain, chills, cyst, flu syndrome, infection, jaw pain, pressure other, jaw tightening, edema of the face, abnormal laboratory test, neck pain, neoplasm, and neck tightness, chest heaviness, chest pain, and chest pressure
Rare: cellulitis, fever, jaw pressure, and neck heaviness
Cardiovascular:
Infrequent: arrhythmias, hypertension, syncope, thrombophlebitis, and tachycardia
Rare: angina pectoris, bradycardia, atrial fibrillation, myocardial infarct, vasodilation, and vascular disorder
Digestive:
Infrequent: diarrhea, dyspepsia, tongue edema, gastrointestinal disorder, increased saliva, and thirst
Rare: increased appetite, colitis, constipation, eructation, gastritis, gastrointestinal carcinoma, gingivitis, hepatic neoplasia, intestinal obstruction, jaundice, sialadenitis, and stomatitis
Endocrine System:
Rare: hyperthyroidism and thyroid edema
Hemic:
Infrequent: cyanosis
Rare: ecchymosis, lymphadenopathy and leukopenia
Metabolic Nutritional:
Rare: increased weight, dehydration, and peripheral edema
Musculoskeletal:
Infrequent: arthralgia, joint disorder, and myalgia
Rare: bone pain, osteoporosis, tenosynovitis and twitching
Nervous System:
Infrequent: agitation, amnesia, anxiety, ataxia, abnormal coordination, confusion, depersonalization, depression, hypertonia, insomnia, nervousness, speech disorder, abnormal thinking, tremor, vertigo, and circumoral paresthesia
Rare: apathy, convulsions, abnormal dreams, euphoria, hypertonia, irritability, tardive dyskinesia, manic reaction, neuropathy, and psychosis
Respiratory:
Infrequent: bronchitis, increased cough, dyspnea, epistaxis, laryngeal edema, pharyngitis, rhinitis, sinusitis, throat discomfort, and voice alteration
Rare: hiccup, hyperventilation, laryngitis, pneumonia, increased sputum, and yawning
Skin:
Infrequent: pruritus, rash, skin disorder, and sweating
Rare: eczema, erythema, erythema multiform, hair disorder, and neoplasm
Special Senses:
Infrequent: amblyopia, disorder of lacrimation, ear pain, eye pain, parosmia and tinnitus
Rare: conjunctivitis, dry eye, photophobia, and visual field defect
Urogenital:
Infrequent: polyuria and menorrhagia
Rare: breast carcinoma, dysmenorrhea, metrorrhagia, breast neoplasm, unintended pregnancy, suspicious PAP smear, uterine disorder, enlarged uterine fibroids, fibrocytic breast, vaginitis, urogenital neoplasm, cystitis, urinary tract infection, kidney pain, pyelonephritis, urinary frequency, urine impaired, and urinary tract disorder
The adverse experience profile seen with ZOMIG Nasal Spray is similar to that seen with ZOMIG tablets and ZOMIG-ZMT tablets except for the occurrence of local adverse reactions from the nasal spray (see ZOMIG Tablet Prescribing Information).
6.2 Postmarketing Experience with ZOMIG Tablets
The following adverse reactions were identified during post approval use of ZOMIG. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following section enumerates potentially important adverse reactions that have occurred in clinical practice and which have been reported spontaneously to various surveillance systems. The reactions enumerated represent reports arising from both domestic and non-domestic use of oral zolmitriptan. The reactions enumerated include all except those already listed in the ADVERSE REACTIONS section above or those too general to be informative. Because the reports cite reactions reported spontaneously from worldwide postmarketing experience, frequency of reactions and the role of zolmitriptan in their causation cannot be reliably determined.
Cardiovascular:
Coronary artery vasospasm, transient myocardial ischemia, angina pectoris, and myocardial infarction.
Digestive:
Very rare gastrointestinal ischemic reactions including splenic infarction, ischemic colitis and gastrointestinal infarction or necrosis have been reported; these may present as bloody diarrhea or abdominal pain [see Warnings and Precautions (5.4)].
General:
As with other 5-HT1B/1D agonists, there have been very rare reports of anaphylaxis or anaphylactoid reactions in patients receiving ZOMIG. There have been rare reports of hypersensitivity reactions, including angioedema.
Serotonin syndrome has also been reported during the postmarketing period [see Warnings and Precautions (5.5)].
Neurological:
As with other acute migraine treatments including other 5HT1 agonists, there have been rare reports of headache.
7 DRUG INTERACTIONS
7.1 Ergot-containing drugs
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because there is a theoretical basis that these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and zolmitriptan within 24 hours of each other should be avoided [see Contraindications (4.5)].
7.2 MAO-A Inhibitors
MAO-A inhibitors increase the systemic exposure of zolmitriptan. Therefore, the use of zolmitriptan in patients receiving MAO-A inhibitors is contraindicated [see Clinical Pharmacology (12.4) and Contraindications (4.7)].
7.3 5-HT agonists (e.g. triptans)
Concomitant use of other 5-HT1B/1D agonists within 24 hours of ZOMIG treatment is not recommended [see Contraindications (4.5)].
7.4 Cimetidine
Following administration of cimetidine, the half-life and AUC of zolmitriptan and its active metabolites were approximately doubled [see Clinical Pharmacology (12.4)].
7.5 Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome
Cases of life-threatening serotonin syndrome have been reported during combined use of selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) and triptans [see Warnings and Precautions (5.5)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. There are no adequate and well controlled studies in pregnant women; therefore, zolmitriptan should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. In reproductive toxicity studies in rats and rabbits, oral administration of zolmitriptan to pregnant animals resulted in embryolethality and fetal abnormalities (malformations and variations) at clinically relevant exposures.
When zolmitriptan was administered to pregnant rats during the period of organogenesis at oral doses of 100, 400, and 1200 mg/kg/day (plasma exposures (AUCs) ≈280, 1100, and 5000 times the human AUC at the maximum recommended human dose (MRHD) of 10 mg/day, there was a dose-related increase in embryolethality. A no-effect dose for embryolethality was not established. When zolmitriptan was administered to pregnant rabbits during the period of organogenesis at oral doses of 3, 10, and 30 mg/kg/day (plasma AUCs ≈1, 11, and 42 times the human AUC at the MRHD), there were increases in embryolethality and in fetal malformations and variations. The no-effect dose for adverse effects on embryo-fetal development was associated with a plasma AUC similar to that in humans at the MRHD. When female rats were given zolmitriptan during gestation, parturition, and lactation at oral doses of 25, 100, and 400 mg/kg/day (plasma AUCs ≈70, 280, and 1100 times that in human at the MRHD), an increased incidence of hydronephrosis was found in the offspring. The no-effect dose was associated with a plasma AUC ≈280 times that in humans at the MRHD.
8.3 Nursing Mothers
It is not known whether zolmitriptan is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when zolmitriptan is administered to a nursing woman. Lactating rats dosed with zolmitriptan had levels in milk equivalent to maternal plasma levels at 1 hour and 4 times higher than plasma levels at 4 hours.
8.4 Pediatric Use
Safety and effectiveness of ZOMIG in pediatric patients have not been established; therefore, ZOMIG is not recommended for use in patients under 18 years of age.
A single, multicenter, double-blind, randomized placebo-controlled, study was conducted to evaluate the efficacy of zolmitriptan 5 mg nasal spray in the acute treatment of migraine headache in 171 evaluable adolescent subjects 12 to 17 years of age. Efficacy was not established in that study.
Adverse reactions observed in this study were similar in nature and frequency to those reported in ZOMIG Nasal Spray adult clinical trials. The most commonly reported adverse reactions (≥ 2% and > placebo) were dysgeusia (7%), nasal discomfort (3%), dizziness (2%), nasal congestion (2%), nausea (2%), and throat irritation (2%).
ZOMIG Nasal Spray has not been studied in pediatric patients under 12 years of age.
In the postmarketing experience with triptans, including ZOMIG, there is a limited number of reports that describe pediatric patients who have experienced clinically serious adverse events; those that were reported are similar in nature to those reported rarely in adults.
8.5 Geriatric Use
Although the pharmacokinetic disposition of the drug in the elderly is similar to that seen in younger adults, there is no information about the safety and effectiveness of zolmitriptan in this population because patients over age 65 were excluded from the controlled clinical trials [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
The effect of hepatic disease on the pharmacokinetics of zolmitriptan nasal spray has not been evaluated. After oral administration, zolmitriptan exposure was increased in patients with severe hepatic impairment, and significant elevation in blood pressure was observed in some patients. Because of the similarity in exposure, zolmitriptan tablets and nasal spray should have similar dosage adjustments and should be administered with caution in subjects with liver disease, generally using doses less than 2.5 mg. Doses lower than 5 mg can only be achieved through the use of an oral formulation [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
The abuse potential of ZOMIG has not been assessed in clinical trials.
10 OVERDOSAGE
There is no experience with acute overdose. Clinical study subjects receiving single 50 mg oral doses of zolmitriptan commonly experienced sedation.
The elimination half-life of ZOMIG is 3 hours [see Clinical Pharmacology (12.1)] and therefore monitoring of patients after overdose with ZOMIG should continue for at least 15 hours or while symptoms or signs persist.
There is no specific antidote to zolmitriptan. In cases of severe intoxication, intensive care procedures are recommended, including establishing and maintaining a patent airway, ensuring adequate oxygenation and ventilation, and monitoring and support of the cardiovascular system.
It is unknown what effect hemodialysis or peritoneal dialysis has on the plasma concentrations of zolmitriptan.
11 DESCRIPTION
ZOMIG® (zolmitriptan) Nasal Spray contains zolmitriptan, which is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist. Zolmitriptan is chemically designated as (S)-4-[[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone and has the following chemical structure:
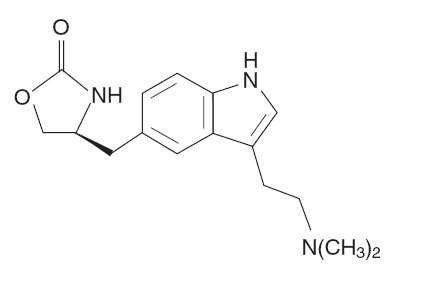
The empirical formula is C16H21N3O2, representing a molecular weight of 287.36. Zolmitriptan is a white to almost white powder that is readily soluble in water. ZOMIG Nasal Spray is supplied as a clear to pale yellow solution of zolmitriptan, buffered to a pH 5.0. Each ZOMIG Nasal Spray contains 5 mg of zolmitriptan in a 100-μL unit dose aqueous buffered solution containing citric acid, anhydrous, USP, disodium phosphate dodecahydrate USP and purified water USP.
ZOMIG Nasal Spray is hypertonic. The osmolarity of ZOMIG Nasal Spray 5 mg is 420 to 470 mOsmol.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zolmitriptan binds with high affinity to human recombinant 5-HT1D and 5-HT1B receptors. Zolmitriptan exhibits modest affinity for 5-HT1A receptors, but has no significant affinity (as measured by radioligand binding assays) or pharmacological activity at 5-HT2, 5-HT3, 5-HT4, α1-, α2- or β1-adrenergic; H1, H2, histaminic; muscarinic; D1, or D2 receptors. The N-desmethyl metabolite also has high affinity for 5-HT1B/1D and modest affinity for 5-HT1A receptors.
Current theories proposed to explain the etiology of migraine headache suggest that symptoms are due to local cranial vasodilatation and/or to the release of sensory neuropeptides (vasoactive intestinal peptide, substance P and calcitonin gene-related peptide) through nerve endings in the trigeminal system. The therapeutic activity of zolmitriptan for the treatment of migraine headache can most likely be attributed to the agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels (including the arterio-venous anastomoses) and sensory nerves of the trigeminal system which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
12.3 Pharmacokinetics
Absorption:
Zolmitriptan nasal spray is rapidly absorbed via the nasopharynx as detected in a Photon Emission Tomography (PET) study using 11C zolmitriptan. Zolmitriptan was detected in plasma by 5 minutes and peak plasma concentration generally was achieved by 3 hours. The time at which maximum plasma concentrations were observed was similar after single (1 day) or multiple (4 day) nasal dosing. Plasma concentrations of zolmitriptan are sustained for 4 to 6 hours after dosing. Zolmitriptan displays linear kinetics after multiple doses of 2.5 mg, 5 mg, or 10 mg. The mean relative bioavailability of the nasal spray formulation is 102%, compared with the oral tablet.
Zolmitriptan and its active metabolite display dose proportionality after single or multiple dosing. Dose proportional increases in zolmitriptan and N-desmethyl metabolite Cmax and AUC were observed for 2.5 and 5 mg nasal spray doses. The pharmacokinetics for elimination of zolmitriptan and its active N-desmethyl metabolite are similar for all nasal spray dosages. The N-desmethyl metabolite is detected in plasma by 15 minutes and peak plasma concentration is generally achieved by 3 hours after administration.
Food has no significant effect on the bioavailability of zolmitriptan.
Distribution:
Plasma protein binding of zolmitriptan is 25% over the concentration range of 10-1000 ng/mL. The mean (±SD) apparent volume of distribution for zolmitriptan nasal spray formulation is 8.4±3.3 L/kg.
Metabolism:
Zolmitriptan is converted to an active N-desmethyl metabolite such that the metabolite concentrations are about two-thirds that of zolmitriptan. Because the 5HT1B/1D potency of the metabolite is 2 to 6 times that of the parent compound, the metabolite may contribute a substantial portion of the overall effect after zolmitriptan administration.
Excretion:
The mean elimination half-life for zolmitriptan and its active N-desmethyl metabolite following nasal spray administration are approximately 3 hours, which is similar to the half-life values seen after oral tablet administration. The half-life values were similar for zolmitriptan and the N-desmethyl metabolite after single (1 day) and multiple (4 day) nasal dosing.
Mean total plasma clearance is 25.9 mL/min/kg, of which one-sixth is renal clearance. The renal clearance is greater than the glomerular filtration rate suggesting renal tubular secretion.
Special Populations:
Age:
The pharmacokinetics of oral zolmitriptan in healthy elderly non-migraineur volunteers (age 65 - 76 yrs) was similar to those in younger non-migraineur volunteers (age 18-39 yrs).
Gender:
Mean plasma concentrations of orally administered zolmitriptan were up to 1.5-fold higher in females than males.
Renal Impairment:
The effect of renal impairment on the pharmacokinetics of zolmitriptan nasal spray has not been evaluated. After orally dosing zolmitriptan, renal clearance was reduced by 25% in patients with severe renal impairment (Clcr ≥ 5 ≤ 25 mL/min) compared with the normal group (Clcr ≥ 70 mL/min); no significant change in renal clearance was observed in the moderately renally impaired group (Clcr ≥ 26 ≤ 50 mL/min).
Hepatic Impairment:
The effect of hepatic disease on the pharmacokinetics of zolmitriptan nasal spray has not been evaluated. In severely hepatically impaired patients, the mean Cmax, Tmax, and AUC0-∞ of zolmitriptan dosed orally were increased 1.5, 2, and 3-fold, respectively, compared with normals. Seven out of 27 patients experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a 10 mg dose. Because of the similarity in exposure, zolmitriptan tablets and nasal spray should have similar dosage adjustments and should be administered with caution in subjects with liver disease, generally using doses less than 2.5 mg. Doses lower than 5 mg can only be achieved through the use of an oral formulation [see Dosing and Administration (2.2) and Use in Special Populations (8.6) ].
Hypertensive Patients:
No differences in the pharmacokinetics of oral zolmitriptan or its effects on blood pressure were seen in mild to moderate hypertensive volunteers compared with normotensive controls.
Race:
Retrospective analysis of pharmacokinetic data between Japanese and Caucasians revealed no significant differences for orally dosed zolmitriptan.
12.4 Drug Interactions
All drug interaction studies were performed in healthy volunteers using a single 10 mg dose of zolmitriptan and a single dose of the other drug except where otherwise noted. Eight drug interaction studies have been performed with zolmitriptan tablets and one study (xylometazoline) was performed with nasal spray.
Xylometazoline:
An in vivo drug interaction study with ZOMIG Nasal Spray indicated that 1 spray (100μL dose) of xylometazoline (0.1% w/v), a decongestant, administered 30 minutes prior to a 5 mg nasal dose of zolmitriptan did not alter the pharmacokinetics of zolmitriptan.
Fluoxetine:
The pharmacokinetics of zolmitriptan, as well as its effect on blood pressure, were unaffected by 4 weeks of pre-treatment with oral fluoxetine (20 mg/day).
MAO Inhibitors:
Following one week of administration of 150 mg bid moclobemide, a specific MAO-A inhibitor, there was an increase of about 25% in both Cmax and AUC for zolmitriptan and a 3-fold increase in the Cmax and AUC of the active N-desmethyl metabolite of zolmitriptan [see Contraindications (4) and Warnings and Precautions (5)].
Selegiline, a selective MAO-B inhibitor, at a dose of 10 mg/day for 1 week, had no effect on the pharmacokinetics of zolmitriptan and its metabolite.
Propranolol:
Cmax and AUC of zolmitriptan increased 1.5-fold after one week of dosing with propranolol (160 mg/day). Cmax and AUC of the N-desmethyl metabolite were reduced by 30% and 15%, respectively. There were no interactive effects on blood pressure or pulse rate following administration of propranolol with zolmitriptan.
Acetaminophen:
A single 1 g dose of acetaminophen does not alter the pharmacokinetics of zolmitriptan and its N-desmethyl metabolite. However, zolmitriptan delayed the Tmax of acetaminophen by one hour.
Metoclopramide:
A single 10 mg dose of metoclopramide had no effect on the pharmacokinetics of zolmitriptan or its metabolites.
Oral Contraceptives:
Retrospective analysis of pharmacokinetic data across studies indicated that mean plasma concentrations of zolmitriptan were generally higher in females taking oral contraceptives compared with those not taking oral contraceptives. Mean Cmax and AUC of zolmitriptan were found to be higher by 30% and 50%, respectively, and Tmax was delayed by one-half hour in females taking oral contraceptives. The effect of zolmitriptan on the pharmacokinetics of oral contraceptives has not been studied.
Cimetidine:
Following the administration of cimetidine, the half-life and AUC of a 5 mg dose of zolmitriptan and its active metabolite were approximately doubled [see Drug Interactions (7.4)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Zolmitriptan was administered to mice and rats at doses up to 400 mg/kg/day. Mice were dosed for 85 weeks (males) and 92 weeks (females); rats were dosed for 101 weeks (males) and 86 weeks (females). There was no evidence of drug-induced tumors in mice at plasma exposures (AUC) up to approximately 700 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day. In rats, there was an increase in the incidence of thyroid follicular cell hyperplasia and thyroid follicular cell adenomas seen in male rats receiving 400 mg/kg/day. The no-effect dose for tumors in rats (100 mg/kg/day) was associated with a plasma AUC ≈700 times that in humans at the MRHD.
13.2 Animal Toxicology and/or Pharmacology
Zolmitriptan was positive in an in vitro bacterial reverse mutation (Ames) assay and in an in vitro chromosomal aberration assay in human lymphocytes. Zolmitriptan was negative in an in vitro mammalian gene cell mutation (CHO/HGPRT) assay and in oral in vivo micronucleus assays in mouse and rat.
13.3 Impairment of Fertility
Studies of male and female rats administered zolmitriptan prior to and during mating and up to implantation showed no impairment of fertility at oral doses up to 400 mg/kg/day. The plasma exposure (AUC) at this dose was approximately 3000 times that in humans at the maximum recommended human dose of 10 mg/day.
14 CLINICAL STUDIES
The efficacy of ZOMIG Nasal Spray 5 mg in the acute treatment of migraine headache with or without aura was demonstrated in a randomized, outpatient, double-blind, placebo-controlled trial.
Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed 15, 30, 45 minutes and 1, 2, and 4 hours after dosing. Pain free response rates and associated symptoms such as nausea, photophobia, and phonophobia were also assessed. A dose of escape medication was allowed 4 to 24 hours after the initial treatment for persistent and recurrent headache.
Of the 1372 patients treated in the study, 83% were female and 99% were Caucasian, with a mean age of 40.6 years (range 18 to 65 years).
The two hour headache response rates in patients treated with ZOMIG Nasal Spray were statistically significant among patients receiving ZOMIG Nasal Spray compared with placebo. There was a greater percentage of patients with a headache response at 2 hours in the higher dose groups. The headache response efficacy endpoints of the controlled clinical study, analyzed from the first attack data, are shown in Table 2.
Table 2: First Attack Data: Percentage of Patients with Headache Response to ZOMIG Nasal Spray (Mild or No Headache) 2 Hours Following Treatment
(N = number of randomized patients treating a migraine attack). The 2 hour headache response wNas the primary end-point.
| N | PLACEBO (226) |
ZOMIG 5 mg (235) |
|
2 hours |
31% |
69% |
The estimated probability of achieving an initial headache response by 4 hours following treatment with ZOMIG Nasal Spray is depicted in Figure 1.
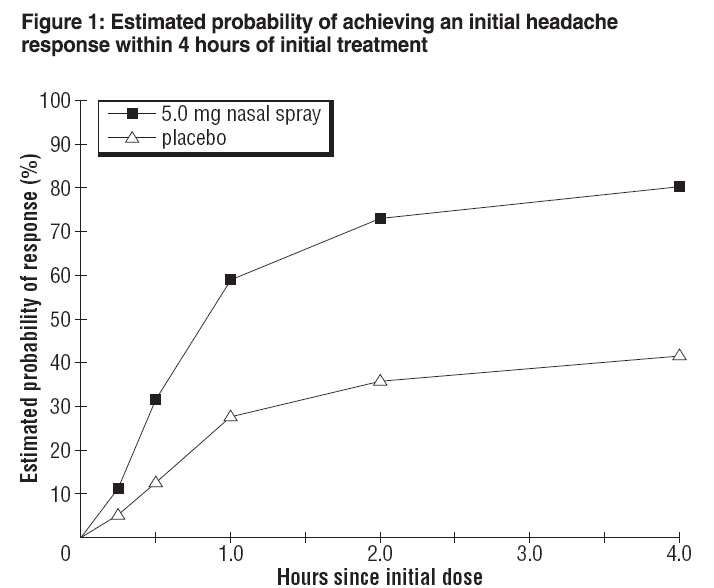
Note:
Figure 1 shows the Kaplan-Meier plot of the probability over time of obtaining headache response (moderate or severe headache improving to mild or no pain) following treatment with zolmitriptan nasal spray. The averages displayed are based on a placebo controlled, outpatient trial providing evidence of efficacy. Patients not achieving headache response or taking additional treatment prior to 4 hours were censored to 4 hours.
For patients with migraine associated photophobia, phonophobia, and nausea at baseline, there was a decreased incidence of these symptoms following administration of ZOMIG Nasal Spray as compared with placebo.
Four to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.

*This Kaplan-Meier plot is based on data obtained from the placebo controlled clinical trial. Patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. It should be noted that the protocol did not allow remedication within 4 hours post dose.
The efficacy of ZOMIG was unaffected by presence of aura; presence of headache upon awakening, relationship to menses; gender, age or weight of the patient; or presence of pre-treatment nausea.
The efficacy of ZOMIG Nasal Spray 5 mg was further supported by an interim analysis of another similarly designed trial. The 2 hour headache response rates for the first 210 subjects in that study for ZOMIG 5 mg and placebo were 70% and 47%, respectively (N=108 and 102, respectively, p=0.0006).
16 HOW SUPPLIED/STORAGE AND HANDLING
The ZOMIG Nasal Spray device is a blue colored plastic device with a gray protection cap, labeled to indicate the nominal dose. Each ZOMIG Nasal Spray device administers a single dose of ZOMIG.
ZOMIG Nasal Spray is supplied as a clear to pale yellow solution of zolmitriptan, buffered to a pH 5.0. Each ZOMIG Nasal Spray device contains 5 mg of zolmitriptan in a 100-μL unit dose aqueous buffered solution containing citric acid, anhydrous, USP, disodium phosphate dodecahydrate USP and purified water USP.
5 mg ZOMIG® Nasal Spray is supplied in boxes of 6 single use nasal spray units. (NDC 21695-955-06).
Each ZOMIG® Nasal Spray single dose unit spray supplies 5 mg of zolmitriptan. The ZOMIG® Nasal Spray unit must be discarded after use.
Store at controlled room temperature, 20-25°C (68-77°F) [see USP].
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (17.5)
17.1 Risk of Myocardial Ischemia and/or Infarction, Other Adverse Cardiac Events, Other Vasospasm-related Event, and Cerebrovascular Events
Patients should be informed that ZOMIG may cause serious cardiovascular side effects such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up [see Warnings and Precautions (5.1, 5.3, 5.4)].
17.2 Serotonin Syndrome
Patients should be cautioned about the risk of serotonin syndrome with the use of ZOMIG or other triptans, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) [see Warnings and Precautions (5.5)].
17.3 Device Use
The ZOMIG Nasal Spray device is packaged in a carton and is a blue colored plastic device with a gray protection cap, labeled to indicate the nominal dose. Patients should be cautioned to not remove the gray protection cap until prior to dosing. The ZOMIG Nasal Spray device is placed in a nostril and actuated to deliver a single dose. Patients should be cautioned to avoid spraying the contents of the device in their eyes.
17.4 Pregnancy
ZOMIG should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
17.5 Approved Patient Labeling
Please read this information before you start taking ZOMIG Nasal Spray and each time you renew your prescription just in case anything has changed. Remember, this summary does not take the place of discussions with your doctor. You and your doctor should discuss ZOMIG Nasal Spray when you start taking your medication and at regular checkups.
What is ZOMIG Nasal Spray?
ZOMIG Nasal Spray is a prescription medication used to treat migraine headaches in adults. ZOMIG Nasal Spray is not for other types of headaches. The safety and efficacy of ZOMIG in patients under 18 have not been established.
What is a Migraine Headache?
Migraine is an intense, throbbing headache. You may have pain on one or both sides of your head. You may have nausea and vomiting, and be sensitive to light and noise. The pain and symptoms of a migraine headache can be worse than a common headache. Some women get migraines around the time of their menstrual period. Some people have visual symptoms before the headache, such as flashing lights or wavy lines, called an aura.
How does ZOMIG Nasal Spray work?
Treatment with ZOMIG Nasal Spray reduces swelling of blood vessels surrounding the brain. This swelling is associated with the headache pain of a migraine attack. ZOMIG Nasal Spray blocks the release of substances from nerve endings that cause more pain and other symptoms like nausea, and sensitivity to light and sound. It is thought that these actions contribute to relief of your symptoms by ZOMIG Nasal Spray.
Who should not take ZOMIG Nasal Spray?
Do not take ZOMIG Nasal Spray if you:
-
-
-
-
-
-
-
-
Tell your doctor about all the medicines you take or plan to take, including prescription and nonprescription medicines, supplements, and herbal remedies.
Tell your doctor if you are taking selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs), two types of drugs for depression or other disorders. Common SSRIs are CELEXA® (citalopram HBr), LEXAPRO® (escitalopram oxalate), PAXIL® (paroxetine), PROZAC® (fluoxetine), SYMBYAX® (olanzapine/fluoxetine), ZOLOFT® (sertraline), SARAFEM® (fluoxetine) and LUVOX® (fluvoxamine). Common SNRIs are CYMBALTA® (duloxetine) and EFFEXOR® (venlafaxine). Your doctor will decide if you can take ZOMIG Nasal Spray with your other medicines.
Tell your doctor if you know that you have any of the following: risk factors for heart disease like high cholesterol, diabetes, smoking, obesity (overweight), menopause, or a family history of heart disease or stroke.
Tell your doctor if you are pregnant, planning to become pregnant, breast feeding, planning to breast feed, or not using effective birth control.
How should I take ZOMIG NASAL Spray?
The ZOMIG Nasal Spray device is a blue colored plastic sprayer device with a gray protection cap, labeled to indicate the dose. For adults, the usual dose is a single nasal spray taken into one nostril. If your headache comes back after your first dose, you may take a second dose anytime after 2 hours of taking the first dose. For any attack where the first dose didn’t work, do not take a second dose without talking with your doctor. Do not take more than a total of 10 mg of ZOMIG (tablets or spray combined) in any 24-hour period. If you take too much medicine, contact your doctor, hospital emergency department, or poison control center right away.
The ZOMIG Nasal Spray device consists of the following parts:
A. The Tip: This is the part that you put into your nostril. The medicine comes out of a tiny hole in the top.
B. The Protective Cap: This covers the tip to protect it. Do not remove the protective cap until just before you are ready to take your ZOMIG Nasal Spray.
C. The Finger-grip: This is the part that you hold when you use the sprayer.
D. The Plunger: This is the part that you press when you put the tip into your nostril. This sprayer works only once.
Steps for using ZOMIG Nasal Spray (Please read all steps before using for the first time):
1. Blow your nose gently before use. Remove the protective cap (B) (Figure 1). Hold the nasal sprayer device gently with your fingers and thumb as shown in the picture to the right (Figure 2). There is only one dose in the nasal sprayer. Do not try to prime the nasal sprayer or you will lose the dose. Do not press the plunger until you have put the tip into your nostril or you will lose the dose.
2. Block one nostril by pressing firmly on the side of your nose (Figure 3). Either nostril can be used. Put the tip (A) of the sprayer device into the other nostril as far as feels comfortable and tilt your head slightly as shown in the picture to the right (Figure 4).
Do not press the plunger yet.
Do not spray the contents of the device in your eyes.


3. Breathe in gently through your nose and at the same time press the plunger (D) firmly with your thumb. The plunger may feel stiff and you may hear a click. Keep your head slightly tilted back and remove the tip from your nose. Breathe gently through your mouth for 5-10 seconds. You may feel liquid in your nose or the back of your throat. This is normal and will soon pass.
What are the possible side effects of ZOMIG Nasal Spray?
ZOMIG Nasal Spray is generally well tolerated. As with any medicine, people taking ZOMIG Nasal Spray may have side effects. The side effects are usually mild and do not last long.
The most common side effects of ZOMIG Nasal Spray are:
-
-
-
-
-
In very rare cases, patients taking triptans may experience serious side effects, such as heart attacks, high blood pressure, stroke, or serious allergic reactions. Extremely rarely, patients have died. Call your doctor right away if you have any of the following problems after taking ZOMIG Nasal Spray:
-
-
-
-
-
-
Some people may have a reaction called serotonin syndrome, which can be life-threatening, when they use ZOMIG. In particular, this reaction may occur when they use ZOMIG together with certain types of antidepressants known as SSRIs or SNRIs. Symptoms may include mental changes (hallucinations, agitation, coma), fast heartbeat, changes in blood pressure, high body temperature or sweating, tight muscles, trouble walking, nausea, vomiting, and diarrhea. Call your doctor immediately if you have any of these symptoms after taking ZOMIG.
This is not a complete list of side effects. Talk to your doctor if you develop any symptoms that concern you.
What to do in case of an overdose?
Call your doctor or poison control center or go to the ER.
General advice about ZOMIG Nasal Spray
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use ZOMIG Nasal Spray for a condition for which it was not prescribed. Do not give ZOMIG Nasal Spray to other people, even if they have the same symptoms as you. People may be harmed if they take medicines that have not been prescribed for them.
This leaflet summarizes the most important information about ZOMIG Nasal Spray. If you would like more information about ZOMIG Nasal Spray, talk to your doctor. You can ask your doctor or pharmacist for information on ZOMIG Nasal Spray that is written for health professionals. You can also call 1-800-236-9933 or visit our web site at www.ZOMIG.com.
What are the Ingredients in ZOMIG Nasal Spray?
Active ingredient: zolmitriptan
Inactive ingredients: anhydrous citric acid, dibasic sodium phosphate, and purified water
Store your medication at controlled room temperature, 20-25°C (68-77°F), and away from children. Discard after use or when it expires.
ZOMIG is a registered trademark of the AstraZeneca group of companies.
Other brands mentioned are trademarks of their respective owners and are not trademarks of the AstraZeneca group of companies. The makers of these brands are not affiliated with AstraZeneca or its products.
©AstraZeneca 2008
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, Delaware 19850
By: AstraZeneca UK Limited, Macclesfield,
Cheshire UK
Made in the United Kingdom
31455–00
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

ZOMIGZolmitriptan SPRAY, METERED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
