Zosyn
Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZOSYN safely and effectively. See full prescribing information for ZOSYN.ZOSYN (piperacillin/tazobactam) FOR INJECTION: single-dose vialsInitial U.S. approval: 1993MicrobiologyTo reduce the development of drug-resistant bacteria and maintain the effectiveness of ZOSYN and other antibacterial drugs, ZOSYN should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. INDICATIONS AND USAGEZOSYN is a combination penicillin-class antibacterial and β-lactamase inhibitor indicated for treatment of: Intra-abdominal infections (1.1) Skin and skin structure infections (1.2) Female pelvic infections (1.3) Community-acquired pneumonia (1.4) Nosocomial pneumonia (1.5) DOSAGE AND ADMINISTRATION The usual daily dose of ZOSYN for adults is 3.375 g every six hours totaling 13.5 g (12.0 g piperacillin/1.5 g tazobactam) (2.1) Initial presumptive treatment of patients with nosocomial pneumonia should start with ZOSYN at a dosage of 4.5 g every six hours plus an aminoglycoside, totaling 18.0 g (16.0 g piperacillin/2.0 g tazobactam). (2.2) Dosage in patients with renal impairment (≤40 mL/min of CRCL) and dialysis patients should be reduced, based on the degree of actual renal function impairment. (2.3) For children with appendicitis and/or peritonitis the recommended ZOSYN dosage is 100 mg piperacillin/12.5 mg tazobactam per kilogram of body weight, every 8 hours in pediatric patients 9 months of age and older. For pediatric patients 2 to 9 months of age, the recommended dosage is 80 mg piperacillin/10 mg tazobactam per kilogram of body weight, every 8 hours.(2.4) ZOSYN and aminoglycosides should be reconstituted, diluted, and administered separately. Co-administration via Y-site can be done under certain conditions. (2.6) DOSAGE FORMS AND STRENGTHSZOSYN® for Injection: 2.25 g, 3.375 g, and 4.5 g lyophilized powder for reconstitution in single-dose vials. (3)CONTRAINDICATIONSPatients with a history of allergic reactions to any of the penicillins, cephalosporins, or β-lactamase inhibitors. (4)WARNINGS AND PRECAUTIONS Serious hypersensitivity reactions (anaphylactic/anaphylactoid) reactions have been reported in patients receiving ZOSYN. Discontinue ZOSYN if a reaction occurs. (5.1) Serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported (5.2). Discontinue ZOSYN for progressive rashes. Clostridium difficile associated diarrhea: evaluate patients if diarrhea occurs. (5.3) Hematological effects (including bleeding, leukopenia and neutropenia) have occurred. Monitor hematologic tests during prolonged therapy. (5.4) Side EffectsThe most common adverse reactions (incidence >5%) are diarrhea, constipation, nausea, headache and insomnia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS ZOSYN administration can significantly reduce tobramycin concentrations in hemodialysis patients. Monitor tobramycin concentrations in these patients. (7.1) Probenecid prolongs the half-lives of piperacillin and tazobactam and should not be co-administered with ZOSYN unless the benefit outweighs the risk. (7.2) Monitor coagulation parameters in patients receiving ZOSYN and heparin or oral anticoagulants. (7.3) ZOSYN may prolong the neuromuscular blockade of vecuronium and other non-depolarizing muscle relaxants. Monitor for adverse reactions related to neuromuscular blockade(7.4) USE IN SPECIFIC POPULATIONSDosage in patients with renal impairment (≤40 mL/min of CRCL) should be reduced to the degree of actual renal function impairment. (2.3, 8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ZOSYN INDICATIONS AND USAGE
- 2 ZOSYN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ZOSYN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ZOSYN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ZOSYN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL - 3.375 gram Vial Label
- PRINCIPAL DISPLAY PANEL - 10 x 3.375 gram Vial Carton
- PRINCIPAL DISPLAY PANEL - 2.25 gram Vial Label
- PRINCIPAL DISPLAY PANEL - 10 x 2.25 gram Vial Carton
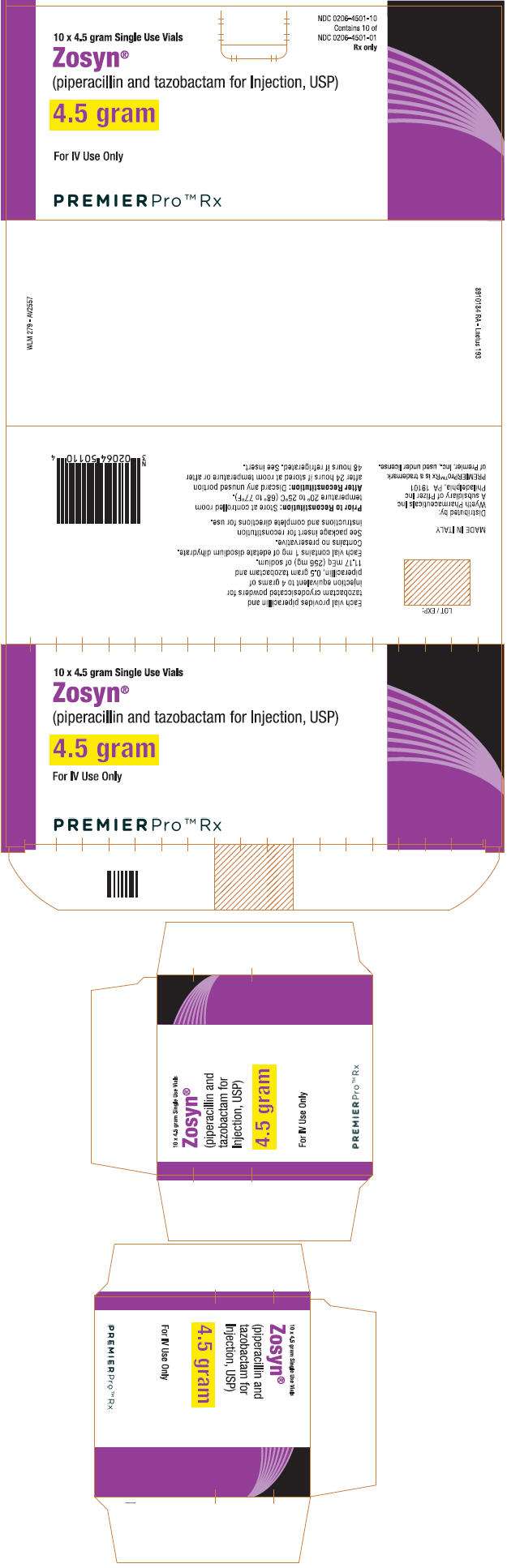
- PRINCIPAL DISPLAY PANEL - 4.5 gram Vial Label
- PRINCIPAL DISPLAY PANEL - 10 x 4.5 gram Vial Carton
- PRINCIPAL DISPLAY PANEL - 40.5 gram Vial Label
- PRINCIPAL DISPLAY PANEL - 40.5 gram Vial Carton
FULL PRESCRIBING INFORMATION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ZOSYN (piperacillin/tazobactam) injection and other antibacterial drugs, ZOSYN (piperacillin/tazobactam) should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
1 INDICATIONS AND USAGE
ZOSYN is a combination product consisting of a penicillin-class antibacterial, piperacillin, and a β-lactamase inhibitor, tazobactam, indicated for the treatment of patients with moderate to severe infections caused by susceptible isolates of the designated bacteria in the conditions listed below.
1.1 Intra-abdominal Infections
Appendicitis (complicated by rupture or abscess) and peritonitis caused by β-lactamase producing isolates of Escherichia coli or the following members of the Bacteroides fragilis group: B. fragilis, B. ovatus, B. thetaiotaomicron, or B. vulgatus. The individual members of this group were studied in fewer than 10 cases.
1.2 Skin and Skin Structure Infections
Uncomplicated and complicated skin and skin structure infections, including cellulitis, cutaneous abscesses and ischemic/diabetic foot infections caused by β-lactamase producing isolates of Staphylococcus aureus.
1.3 Female Pelvic Infections
Postpartum endometritis or pelvic inflammatory disease caused by β-lactamase producing isolates of Escherichia coli.
1.4 Community-acquired Pneumonia
Community-acquired pneumonia (moderate severity only) caused by β-lactamase producing isolates of Haemophilus influenzae.
1.5 Nosocomial Pneumonia
Nosocomial pneumonia (moderate to severe) caused by β-lactamase producing isolates of Staphylococcus aureus and by piperacillin/tazobactam-susceptible Acinetobacter baumannii, Haemophilus influenzae, Klebsiella pneumoniae, and Pseudomonas aeruginosa (Nosocomial pneumonia caused by P. aeruginosa should be treated in combination with an aminoglycoside) [see Dosage and Administration (2) ].
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ZOSYN and other antibacterial drugs, ZOSYN should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
ZOSYN should be administered by intravenous infusion over 30 minutes.
2.1 Adult Patients
The usual total daily dose of ZOSYN for adults is 3.375 g every six hours totaling 13.5 g (12.0 g piperacillin/1.5 g tazobactam). The usual duration of ZOSYN treatment is from 7 to 10 days.
ZOSYN should be administered by intravenous infusion over 30 minutes.
2.2 Nosocomial Pneumonia
Initial presumptive treatment of patients with nosocomial pneumonia should start with ZOSYN at a dosage of 4.5 g every six hours plus an aminoglycoside, totaling 18.0 g (16.0 g piperacillin/2.0 g tazobactam). The recommended duration of ZOSYN treatment for nosocomial pneumonia is 7 to 14 days. Treatment with the aminoglycoside should be continued in patients from whom P. aeruginosa is isolated.
2.3 Renal Impairment
In patients with renal impairment (creatinine clearance ≤ 40 mL/min) and dialysis patients (hemodialysis and CAPD), the intravenous dose of ZOSYN should be reduced to the degree of actual renal function impairment. The recommended daily doses of ZOSYN for patients with renal impairment are as follows:
| Renal Function (creatinine clearance, mL/min) | All Indications (except nosocomial pneumonia) | Nosocomial Pneumonia |
|---|---|---|
| >40 mL/min | 3.375 q 6 h | 4.5 q 6 h |
20–40 mL/min |
2.25 q 6 h | 3.375 q 6 h |
<20 mL/min |
2.25 q 8 h | 2.25 q 6 h |
Hemodialysis |
2.25 q 12 h | 2.25 q 8 h |
| CAPD | 2.25 q 12 h | 2.25 q 8 h |
For patients on hemodialysis, the maximum dose is 2.25 g every twelve hours for all indications other than nosocomial pneumonia and 2.25 g every eight hours for nosocomial pneumonia. Since hemodialysis removes 30% to 40% of the administered dose, an additional dose of 0.75 g ZOSYN (0.67 g piperacillin/0.08 g tazobactam) should be administered following each dialysis period on hemodialysis days. No additional dosage of ZOSYN is necessary for CAPD patients.
2.4 Pediatric Patients
For children with appendicitis and/or peritonitis 9 months of age or older, weighing up to 40 kg, and with normal renal function, the recommended ZOSYN dosage is 100 mg piperacillin/12.5 mg tazobactam per kilogram of body weight, every 8 hours. For pediatric patients between 2 months and 9 months of age, the recommended ZOSYN dosage based on pharmacokinetic modeling, is 80 mg piperacillin/10 mg tazobactam per kilogram of body weight, every 8 hours [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3) ]. Pediatric patients weighing over 40 kg and with normal renal function should receive the adult dose.
It has not been determined how to adjust ZOSYN dosage in pediatric patients with renal impairment.
2.5 Reconstitution and Dilution of Powder Formulations
Single dose vials
Reconstitute ZOSYN vials with a compatible reconstitution diluent from the list provided below.
2.25 g, 3.375 g, and 4.5 g ZOSYN should be reconstituted with 10 mL, 15 mL, and 20 mL, respectively. Swirl until dissolved.
Compatible Reconstitution Diluents for Pharmacy and Single Dose Vials
0.9% sodium chloride for injection
Sterile water for injection
Dextrose 5%
Bacteriostatic saline/parabens
Bacteriostatic water/parabens
Bacteriostatic saline/benzyl alcohol
Bacteriostatic water/benzyl alcohol
Reconstituted ZOSYN solutions for single dose vials should be further diluted (recommended volume per dose of 50 mL to 150 mL) in a compatible intravenous solution listed below. Administer by infusion over a period of at least 30 minutes. During the infusion it is desirable to discontinue the primary infusion solution.
Compatible Intravenous Solutions for Single Dose Vials
0.9% sodium chloride for injection
sterile water for injection
Dextran 6% in saline
Dextrose 5%
Lactated Ringer's Solution (compatible only with reformulated ZOSYN containing EDTA and is compatible for co-administration via a Y-site)
ZOSYN should not be mixed with other drugs in a syringe or infusion bottle since compatibility has not been established.
ZOSYN is not chemically stable in solutions that contain only sodium bicarbonate and solutions that significantly alter the pH.
ZOSYN should not be added to blood products or albumin hydrolysates. Parenteral drug products should be inspected visually for particulate matter or discoloration prior to administration, whenever solution and container permit.
Stability of ZOSYN Powder Formulations Following Reconstitution
ZOSYN reconstituted from single vials is stable in glass and plastic containers (plastic syringes, I.V. bags and tubing) when used with compatible diluents.
Single dose vials should be used immediately after reconstitution. Discard any unused portion after 24 hours if stored at room temperature (20°C to 25°C [68°F to 77°F]), or after 48 hours if stored at refrigerated temperature (2°C to 8°C [36°F to 46°F]). Vials should not be frozen after reconstitution.
Stability studies in the I.V. bags have demonstrated chemical stability (potency, pH of reconstituted solution and clarity of solution) for up to 24 hours at room temperature and up to one week at refrigerated temperature. ZOSYN contains no preservatives. Appropriate consideration of aseptic technique should be used.
ZOSYN reconstituted from single vials can be used in ambulatory intravenous infusion pumps. Stability of ZOSYN in an ambulatory intravenous infusion pump has been demonstrated for a period of 12 hours at room temperature. Each dose was reconstituted and diluted to a volume of 37.5 mL or 25 mL. One-day supplies of dosing solution were aseptically transferred into the medication reservoir (I.V. bags or cartridge). The reservoir was fitted to a preprogrammed ambulatory intravenous infusion pump per the manufacturer's instructions. Stability of ZOSYN is not affected when administered using an ambulatory intravenous infusion pump.
2.6 Compatibility with Aminoglycosides
Due to the in vitro inactivation of aminoglycosides by piperacillin, ZOSYN and aminoglycosides are recommended for separate administration. ZOSYN and aminoglycosides should be reconstituted, diluted, and administered separately when concomitant therapy with aminoglycosides is indicated [see Drug Interactions (7.1) ].
In circumstances where co-administration via Y-site is necessary, ZOSYN formulations containing EDTA are compatible for simultaneous coadministration via Y-site infusion only with the following aminoglycosides under the following conditions:
| Aminoglycoside |
ZOSYN Dose (grams) |
ZOSYN Diluent Volume |
Aminoglycoside Concentration Range |
Acceptable Diluents |
|---|---|---|---|---|
| Amikacin | 2.25, 3.375, 4.5 | 50, 100, 150 | 1.75 – 7.5 | 0.9% sodium chloride or 5% dextrose |
| Gentamicin | 2.25 3.375 4.5 |
50, 100 150 |
0.7 – 3.32 | 0.9% sodium chloride or 5% dextrose |
Only the concentration and diluents for amikacin or gentamicin with the dosages of ZOSYN listed above have been established as compatible for coadministration via Y-site infusion. Simultaneous coadministration via Y-site infusion in any manner other than listed above may result in inactivation of the aminoglycoside by ZOSYN.
ZOSYN is not compatible with tobramycin for simultaneous coadministration via Y-site infusion. Compatibility of ZOSYN with other aminoglycosides has not been established.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
3 DOSAGE FORMS AND STRENGTHS
ZOSYN® (piperacillin and tazobactam) for Injection is supplied as a white to off-white powder in vials of the following sizes:
Each ZOSYN 2.25 g vial provides piperacillin sodium equivalent to 2 grams of piperacillin and tazobactam sodium equivalent to 0.25 g of tazobactam.
Each ZOSYN 3.375 g vial provides piperacillin sodium equivalent to 3 grams of piperacillin and tazobactam sodium equivalent to 0.375 g of tazobactam.
Each ZOSYN 4.5 g vial provides piperacillin sodium equivalent to 4 grams of piperacillin and tazobactam sodium equivalent to 0.5 g of tazobactam.
4 CONTRAINDICATIONS
ZOSYN is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or β-lactamase inhibitors.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with ZOSYN. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. Before initiating therapy with ZOSYN, careful inquiry should be made concerning previous hypersensitivity reactions. If an allergic reaction occurs, ZOSYN should be discontinued and appropriate therapy instituted.
5.2 Serious Skin Reactions
Serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in patients receiving ZOSYN. If patients develop a skin rash they should be monitored closely and ZOSYN discontinued if lesions progress.
5.3 Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ZOSYN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Hematologic Effects
Bleeding manifestations have occurred in some patients receiving β-lactam drugs, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, ZOSYN should be discontinued and appropriate therapy instituted.
The leukopenia/neutropenia associated with ZOSYN administration appears to be reversible and most frequently associated with prolonged administration.
Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy, ie, ≥ 21 days [see Adverse Reactions (6.1) ].
5.5 Central Nervous System Effects
As with other penicillins, patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously (particularly in the presence of renal failure).
5.6 Electrolyte Effects
ZOSYN contains a total of 2.79 mEq (64 mg) of Na+ per gram of piperacillin in the combination product. This should be considered when treating patients requiring restricted salt intake. Periodic electrolyte determinations should be performed in patients with low potassium reserves, and the possibility of hypokalemia should be kept in mind with patients who have potentially low potassium reserves and who are receiving cytotoxic therapy or diuretics.
5.7 Development of Drug-Resistant Bacteria
Prescribing ZOSYN in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During the initial clinical investigations, 2621 patients worldwide were treated with ZOSYN in phase 3 trials. In the key North American monotherapy clinical trials (n=830 patients), 90% of the adverse events reported were mild to moderate in severity and transient in nature. However, in 3.2% of the patients treated worldwide, ZOSYN was discontinued because of adverse events primarily involving the skin (1.3%), including rash and pruritus; the gastrointestinal system (0.9%), including diarrhea, nausea, and vomiting; and allergic reactions (0.5%).
| System Organ Class Adverse Reaction |
|---|
| Gastrointestinal disorders |
| Diarrhea (11.3%) |
| Constipation (7.7%) |
| Nausea (6.9%) |
| Vomiting (3.3%) |
| Dyspepsia (3.3%) |
| Abdominal pain (1.3%) |
| Pseudomembranous colitis (≤1%) |
| General disorders and administration site conditions |
| Fever (2.4%) |
| Injection site reaction (≤1%) |
| Rigors (≤1%) |
| Immune system disorders |
| Anaphylaxis (≤1%) |
| Infections and infestations |
| Candidiasis (1.6%) |
| Metabolism and nutrition disorders |
| Hypoglycemia (≤1%) |
| Musculoskeletal and connective tissue disorders |
| Myalgia(≤1%) |
| Arthralgia (≤1%) |
| Nervous system disorders |
| Headache (7.7%) |
| Insomnia (6.6%) |
| Skin and subcutaneous tissue disorders |
| Rash (4.2%, including maculopapular, bullous, and urticarial) |
| Pruritus (3.1%) |
| Vascular disorders |
| Phlebitis (1.3%) |
| Thrombophlebitis (≤1%) |
| Hypotension (≤1%) |
| Purpura (≤1%) |
| Epistaxis (≤1%) |
| Flushing (≤1%) |
Nosocomial Pneumonia Trials
Two trials of nosocomial lower respiratory tract infections were conducted. In one study, 222 patients were treated with ZOSYN in a dosing regimen of 4.5 g every 6 hours in combination with an aminoglycoside and 215 patients were treated with imipenem/cilastatin (500 mg/500 mg q6h) in combination with an aminoglycoside. In this trial, treatment-emergent adverse events were reported by 402 patients, 204 (91.9%) in the piperacillin/tazobactam group and 198 (92.1%) in the imipenem/cilastatin group. Twenty-five (11.0%) patients in the piperacillin/tazobactam group and 14 (6.5%) in the imipenem/cilastatin group (p > 0.05) discontinued treatment due to an adverse event.
The second trial used a dosing regimen of 3.375 g given every 4 hours with an aminoglycoside.
| System Organ Class Adverse Reaction |
|---|
| Blood and lymphatic system disorders |
| Thrombocythemia (1.4%) |
| Anemia (≤1%) |
| Thrombocytopenia (≤1%) |
| Eosinophilia (≤1%) |
| Gastrointestinal disorders |
| Diarrhea (20%) |
| Constipation (8.4%) |
| Nausea (5.8%) |
| Vomiting (2.7%) |
| Dyspepsia (1.9%) |
| Abdominal pain (1.8%) |
| Stomatitis (≤1%) |
| General disorders and administration site conditions |
| Fever (3.2%) |
| Injection site reaction (≤1%) |
| Infections and infestations |
| Oral candidiasis (3.9%) |
| Candidiasis (1.8%) |
| Investigations |
| BUN increased (1.8%) |
| Blood creatinine increased (1.8%) |
| Liver function test abnormal (1.4%) |
| Alkaline phosphatase increased (≤1%) |
| Aspartate aminotransferase increased (≤1%) |
| Alanine aminotransferase increased (≤1%) |
| Metabolism and nutrition disorders |
| Hypoglycemia (≤1%) |
| Hypokalemia (≤1%) |
| Nervous system disorders |
| Headache (4.5%) |
| Insomnia (4.5%) |
| Renal and urinary disorders |
| Renal failure (≤1%) |
| Skin and subcutaneous tissue disorders |
| Rash (3.9%) |
| Pruritus (3.2%) |
| Vascular disorders |
| Thrombophlebitis (1.3%) |
| Hypotension (1.3%) |
Pediatrics
Studies of ZOSYN in pediatric patients suggest a similar safety profile to that seen in adults. In a prospective, randomized, comparative, open-label clinical trial of pediatric patients with severe intra-abdominal infections (including appendicitis and/or peritonitis), 273 patients were treated with ZOSYN (112.5 mg/kg every 8 hours) and 269 patients were treated with cefotaxime (50 mg/kg) plus metronidazole (7.5 mg/kg) every 8 hours. In this trial, treatment-emergent adverse events were reported by 146 patients, 73 (26.7%) in the ZOSYN group and 73 (27.1%) in the cefotaxime/metronidazole group. Six patients (2.2%) in the ZOSYN group and 5 patients (1.9%) in the cefotaxime/metronidazole group discontinued due to an adverse event.
Adverse Laboratory Events (Seen During Clinical Trials)
Of the trials reported, including that of nosocomial lower respiratory tract infections in which a higher dose of ZOSYN was used in combination with an aminoglycoside, changes in laboratory parameters include:
Hematologic—decreases in hemoglobin and hematocrit, thrombocytopenia, increases in platelet count, eosinophilia, leukopenia, neutropenia. These patients were withdrawn from therapy; some had accompanying systemic symptoms (e.g., fever, rigors, chills).
Coagulation—positive direct Coombs' test, prolonged prothrombin time, prolonged partial thromboplastin time
Hepatic—transient elevations of AST (SGOT), ALT (SGPT), alkaline phosphatase, bilirubin
Renal—increases in serum creatinine, blood urea nitrogen
Additional laboratory events include abnormalities in electrolytes (i.e., increases and decreases in sodium, potassium, and calcium), hyperglycemia, decreases in total protein or albumin, blood glucose decreased, gamma-glutamyltransferase increased, hypokalemia, and bleeding time prolonged.
6.2 Post-Marketing Experience
In addition to the adverse drug reactions identified in clinical trials in Table 3 and Table 4, the following adverse reactions have been identified during postapproval use of ZOSYN.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish causal relationship to drug exposure.
Gastrointestinal—hepatitis, jaundice
Hematologic—hemolytic anemia, agranulocytosis, pancytopenia
Immune—hypersensitivity reactions, anaphylactic/anaphylactoid reactions (including shock)
Renal—interstitial nephritis
Skin and Appendages—erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis
6.3 Additional Experience with piperacillin
The following adverse reaction has also been reported for piperacillin for injection:
Skeletal—prolonged muscle relaxation [see Drug Interactions (7.4)].
Post-marketing experience with ZOSYN in pediatric patients suggests a similar safety profile to that seen in adults.
7 DRUG INTERACTIONS
7.1 Aminoglycosides
Piperacillin may inactivate aminoglycosides by converting them to microbiologically inert amides.
In vivo inactivation:
When aminoglycosides are administered in conjunction with piperacillin to patients with end-stage renal disease requiring hemodialysis, the concentrations of the aminoglycosides (especially tobramycin) may be significantly reduced and should be monitored.
Sequential administration of ZOSYN and tobramycin to patients with either normal renal function or mild to moderate renal impairment has been shown to modestly decrease serum concentrations of tobramycin but no dosage adjustment is considered necessary.
In vitro inactivation:
Due to the in vitro inactivation of aminoglycosides by piperacillin, ZOSYN and aminoglycosides are recommended for separate administration. ZOSYN and aminoglycosides should be reconstituted, diluted, and administered separately when concomitant therapy with aminoglycosides is indicated. ZOSYN, which contains EDTA, is compatible with amikacin and gentamicin for simultaneous Y-site infusion in certain diluents and at specific concentrations. ZOSYN is not compatible with tobramycin for simultaneous Y-site infusion [see Dosage and Administration (2.7)].
7.2 Probenecid
Probenecid administered concomitantly with ZOSYN prolongs the half-life of piperacillin by 21% and that of tazobactam by 71% because probenecid inhibits tubular renal secretion of both piperacillin and tazobactam. Probenecid should not be co-administered with ZOSYN unless the benefit outweighs the risk.
7.3 Anticoagulants
Coagulation parameters should be tested more frequently and monitored regularly during simultaneous administration of high doses of heparin, oral anticoagulants, or other drugs that may affect the blood coagulation system or the thrombocyte function. [see Warnings and Precautions (5.4) ]
7.4 Vecuronium
Piperacillin when used concomitantly with vecuronium has been implicated in the prolongation of the neuromuscular blockade of vecuronium. ZOSYN could produce the same phenomenon if given along with vecuronium. Due to their similar mechanism of action, it is expected that the neuromuscular blockade produced by any of the non-depolarizing muscle relaxants could be prolonged in the presence of piperacillin. Monitor for adverse reactions related to neuromuscular blockade (See package insert for vecuronium bromide).
7.5 Methotrexate
Limited data suggests that co-administration of methotrexate and piperacillin may reduce the clearance of methotrexate due to competition for renal secretion. The impact of tazobactam on the elimination of methotrexate has not been evaluated. If concurrent therapy is necessary, serum concentrations of methotrexate as well as the signs and symptoms of methotrexate toxicity should be frequently monitored.
7.6 Effects on Laboratory Tests
There have been reports of positive test results using the Bio-Rad Laboratories Platelia Aspergillus EIA test in patients receiving piperacillin/tazobactam injection who were subsequently found to be free of Aspergillus infection. Cross-reactions with non-Aspergillus polysaccharides and polyfuranoses with the Bio-Rad Laboratories Platelia Aspergillus EIA test have been reported. Therefore, positive test results in patients receiving piperacillin/tazobactam should be interpreted cautiously and confirmed by other diagnostic methods.
As with other penicillins, the administration of ZOSYN may result in a false-positive reaction for glucose in the urine using a copper-reduction method (CLINITEST®). It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects—Pregnancy Category B
Piperacillin/tazobactam
Teratology studies have been performed in mice and rats and have revealed no evidence of harm to the fetus when piperacillin/tazobactam is administered intravenously up to a dose of 3000/750 mg/kg piperacillin/tazobactam which is 1 to 2 times and 2 to 3 times the human dose of piperacillin and tazobactam, respectively, based on body-surface area (mg/m2).
Piperacillin and tazobactam cross the placenta in humans.
There are, however, no adequate and well-controlled studies with the piperacillin/tazobactam combination or with piperacillin or tazobactam alone in pregnant women. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Piperacillin is excreted in low concentrations in human milk; tazobactam concentrations in human milk have not been studied. Caution should be exercised when ZOSYN is administered to a nursing woman.
8.4 Pediatric Use
Use of ZOSYN in pediatric patients 2 months of age or older with appendicitis and/or peritonitis is supported by evidence from well-controlled studies and pharmacokinetic studies in adults and in pediatric patients. This includes a prospective, randomized, comparative, open-label clinical trial with 542 pediatric patients 2–12 years of age with complicated intra-abdominal infections, in which 273 pediatric patients received piperacillin/tazobactam. Safety and efficacy in pediatric patients less than 2 months of age have not been established [see Clinical Pharmacology (12) and Dosage and Administration (2) ].
It has not been determined how to adjust ZOSYN dosage in pediatric patients with renal impairment.
8.5 Geriatric Use
Patients over 65 years are not at an increased risk of developing adverse effects solely because of age. However, dosage should be adjusted in the presence of renal impairment [see Dosage and Administration (2) ].
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ZOSYN contains 64 mg (2.79 mEq) of sodium per gram of piperacillin in the combination product. At the usual recommended doses, patients would receive between 768 and 1024 mg/day (33.5 and 44.6 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
In patients with creatinine clearance ≤ 40 mL/min and dialysis patients (hemodialysis and CAPD), the intravenous dose of ZOSYN should be reduced to the degree of renal function impairment [see Dosage and Administration (2) ].
8.7 Hepatic Impairment
Dosage adjustment of ZOSYN is not warranted in patients with hepatic cirrhosis [See Clinical Pharmacology (12.3) ].
8.8 Patients with Cystic Fibrosis
As with other semisynthetic penicillins, piperacillin therapy has been associated with an increased incidence of fever and rash in cystic fibrosis patients.
10 OVERDOSAGE
There have been postmarketing reports of overdose with piperacillin/tazobactam. The majority of those events experienced, including nausea, vomiting, and diarrhea, have also been reported with the usual recommended dosages. Patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously (particularly in the presence of renal failure) [see Warnings and Precautions (5.5) ].
Treatment should be supportive and symptomatic according the patient's clinical presentation. Excessive serum concentrations of either piperacillin or tazobactam may be reduced by hemodialysis. Following a single 3.375 g dose of piperacillin/tazobactam, the percentage of the piperacillin and tazobactam dose removed by hemodialysis was approximately 31% and 39%, respectively [see Clinical Pharmacology (12) ].
11 DESCRIPTION
ZOSYN (piperacillin and tazobactam) for Injection and ZOSYN (piperacillin and tazobactam) Injection are injectable antibacterial combination products consisting of the semisynthetic antibacterial piperacillin sodium and the β-lactamase inhibitor tazobactam sodium for intravenous administration.
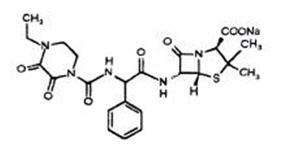
Piperacillin sodium is derived from D(-)-α-aminobenzyl-penicillin. The chemical name of piperacillin sodium is sodium (2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazine-carboxamido)-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate. The chemical formula is C23H26N5NaO7S and the molecular weight is 539.5. The chemical structure of piperacillin sodium is:
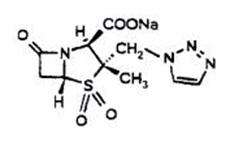
Tazobactam sodium, a derivative of the penicillin nucleus, is a penicillanic acid sulfone. Its chemical name is sodium (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate-4,4-dioxide. The chemical formula is C10H11N4NaO5S and the molecular weight is 322.3. The chemical structure of tazobactam sodium is:
ZOSYN (piperacillin and tazobactam) for Injection, is a white to off-white sterile, cryodesiccated powder consisting of piperacillin and tazobactam as their sodium salts packaged in glass vials. The formulation also contains edetate disodium dihydrate (EDTA) and sodium citrate.
Each ZOSYN 2.25 g single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 2 grams of piperacillin and tazobactam sodium equivalent to 0.25 g of tazobactam. The product also contains 0.5 mg of EDTA per vial.
Each ZOSYN 3.375 g single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 3 grams of piperacillin and tazobactam sodium equivalent to 0.375 g of tazobactam. The product also contains 0.75 mg of EDTA per vial.
Each ZOSYN 4.5 g single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 4 grams of piperacillin and tazobactam sodium equivalent to 0.5 g of tazobactam. The product also contains 1 mg of EDTA per vial.
ZOSYN contains a total of 2.79 mEq (64 mg) of sodium (Na+) per gram of piperacillin in the combination product.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ZOSYN is an antibacterial drug [see Microbiology (12.4) ].
12.2 Pharmacodynamics
The pharmacodynamic parameter for piperacillin/tazobactam that is most predictive of clinical and microbiological efficacy is time above MIC.
12.3 Pharmacokinetics
The mean and coefficients of variation (CV%) for the pharmacokinetic parameters of piperacillin and tazobactam after multiple intravenous doses are summarized in Table 5.
| Piperacillin | ||||||
|---|---|---|---|---|---|---|
Piperacillin/Tazobactam Dose |
Cmax mcg/mL | AUC |
CL mL/min | V L |
T1/2
h |
CLR mL/min |
| 2.25 g | 134 | 131 (14) | 257 | 17.4 | 0.79 | -- |
| 3.375 g | 242 | 242 (10) | 207 | 15.1 | 0.84 | 140 |
| 4.5 g | 298 | 322 (16) | 210 | 15.4 | 0.84 | -- |
| Tazobactam | ||||||
Piperacillin/Tazobactam Dose |
Cmax mcg/mL | AUC |
CL mL/min | V L |
T1/2 h | CLR mL/min |
| 2.25 g | 15 | 16.0 (21) | 258 | 17.0 | 0.77 | -- |
| 3.375 g | 24 | 25.0 (8) | 251 | 14.8 | 0.68 | 166 |
| 4.5 g | 34 | 39.8 (15) | 206 | 14.7 | 0.82 | -- |
Peak plasma concentrations of piperacillin and tazobactam are attained immediately after completion of an intravenous infusion of ZOSYN. Piperacillin plasma concentrations, following a 30-minute infusion of ZOSYN, were similar to those attained when equivalent doses of piperacillin were administered alone. Steady-state plasma concentrations of piperacillin and tazobactam were similar to those attained after the first dose due to the short half-lives of piperacillin and tazobactam.
Distribution
Both piperacillin and tazobactam are approximately 30% bound to plasma proteins. The protein binding of either piperacillin or tazobactam is unaffected by the presence of the other compound. Protein binding of the tazobactam metabolite is negligible.
Piperacillin and tazobactam are widely distributed into tissues and body fluids including intestinal mucosa, gallbladder, lung, female reproductive tissues (uterus, ovary, and fallopian tube), interstitial fluid, and bile. Mean tissue concentrations are generally 50% to 100% of those in plasma. Distribution of piperacillin and tazobactam into cerebrospinal fluid is low in subjects with non-inflamed meninges, as with other penicillins (see Table 6).
| Tissue or Fluid | N |
Sampling period (h) |
Mean PIP Concentration Range (mg/L) |
Tissue:Plasma Range | Tazo Concentration Range (mg/L) |
Tazo Tissue:Plasma Range |
|---|---|---|---|---|---|---|
| Skin | 35 | 0.5 – 4.5 | 34.8 – 94.2 | 0.60 – 1.1 | 4.0 – 7.7 | 0.49 – 0.93 |
| Fatty Tissue | 37 | 0.5 – 4.5 | 4.0 – 10.1 | 0.097 – 0.115 | 0.7 – 1.5 | 0.10 – 0.13 |
| Muscle | 36 | 0.5 – 4.5 | 9.4 – 23.3 | 0.29 – 0.18 | 1.4 – 2.7 | 0.18 – 0.30 |
| Proximal Intestinal Mucosa | 7 | 1.5 – 2.5 | 31.4 | 0.55 | 10.3 | 1.15 |
| Distal Intestinal Mucosa |
7 | 1.5 – 2.5 | 31.2 | 0.59 | 14.5 | 2.1 |
| Appendix | 22 | 0.5 – 2.5 | 26.5 – 64.1 | 0.43 – 0.53 | 9.1 – 18.6 | 0.80 – 1.35 |
Metabolism
Piperacillin is metabolized to a minor microbiologically active desethyl metabolite. Tazobactam is metabolized to a single metabolite that lacks pharmacological and antibacterial activities.
Excretion
Following single or multiple ZOSYN doses to healthy subjects, the plasma half-life of piperacillin and of tazobactam ranged from 0.7 to 1.2 hours and was unaffected by dose or duration of infusion.
Both piperacillin and tazobactam are eliminated via the kidney by glomerular filtration and tubular secretion. Piperacillin is excreted rapidly as unchanged drug with 68% of the administered dose excreted in the urine. Tazobactam and its metabolite are eliminated primarily by renal excretion with 80% of the administered dose excreted as unchanged drug and the remainder as the single metabolite. Piperacillin, tazobactam and desethyl piperacillin are also secreted into the bile.
Specific Populations
Renal impairment
After the administration of single doses of piperacillin/tazobactam to subjects with renal impairment, the half-life of piperacillin and of tazobactam increases with decreasing creatinine clearance. At creatinine clearance below 20 mL/min, the increase in half-life is twofold for piperacillin and fourfold for tazobactam compared to subjects with normal renal function. Dosage adjustments for ZOSYN are recommended when creatinine clearance is below 40 mL/min in patients receiving the usual recommended daily dose of ZOSYN. See Dosage and Administration (2) for specific recommendations for the treatment of patients with renal -impairment.
Hemodialysis removes 30% to 40% of a piperacillin/tazobactam dose with an additional 5% of the tazobactam dose removed as the tazobactam metabolite. Peritoneal dialysis removes approximately 6% and 21% of the piperacillin and tazobactam doses, respectively, with up to 16% of the tazobactam dose removed as the tazobactam metabolite. For dosage recommendations for patients undergoing hemodialysis [see Dosage and Administration (2) .
Hepatic Impairment
The half-life of piperacillin and of tazobactam increases by approximately 25% and 18%, respectively, in patients with hepatic cirrhosis compared to healthy subjects. However, this difference does not warrant dosage adjustment of ZOSYN due to hepatic cirrhosis.
Pediatrics
Piperacillin and tazobactam pharmacokinetics were studied in pediatric patients 2 months of age and older. The clearance of both compounds is slower in the younger patients compared to older children and adults.
In a population PK analysis, estimated clearance for 9 month-old to 12 year-old patients was comparable to adults, with a population mean (SE) value of 5.64 (0.34) mL/min/kg. The piperacillin clearance estimate is 80% of this value for pediatric patients 2 – 9 months old. In patients younger than 2 months of age, clearance of piperacillin is slower compared to older children; however, it is not adequately characterized for dosing recommendations. The population mean (SE) for piperacillin distribution volume is 0.243 (0.011) L/kg and is independent of age.
Geriatrics
The impact of age on the pharmacokinetics of piperacillin and tazobactam was evaluated in healthy male subjects, aged 18 – 35 years (n=6) and aged 65 to 80 years (n=12). Mean half-life for piperacilln and tazobactam was 32% and 55% higher, respectively, in the elderly compared to the younger subjects. This difference may be due to age-related changes in creatinine clearance.
Race
The effect of race on piperacillin and tazobactam was evaluated in healthy male volunteers. No difference in piperacillin or tazobactam pharmacokinetics was observed between Asian (n=9) and Caucasian (n=9) healthy volunteers who received single 4/0.5 g doses.
Drug Interactions
The potential for pharmacokinetic drug interactions between ZOSYN and aminoglycosides, probenecid, vancomycin, heparin, vecuronium, and methotrexate has been evaluated [see Drug Interactions (7) ].
12.4 Microbiology
Mechanism of Action
Piperacillin sodium exerts bactericidal activity by inhibiting septum formation and cell wall synthesis of susceptible bacteria. In vitro, piperacillin is active against a variety of Gram-positive and Gram-negative aerobic and anaerobic bacteria. Tazobactam sodium has little clinically relevant in vitro activity against bacteria due to its reduced affinity to penicillin-binding proteins. It is, however, a β-lactamase inhibitor of the Molecular class A enzymes, including Richmond-Sykes class III (Bush class 2b & 2b') penicillinases and cephalosporinases. It varies in its ability to inhibit class II and IV (2a & 4) penicillinases. Tazobactam does not induce chromosomally-mediated β-lactamases at tazobactam concentrations achieved with the recommended dosage regimen.
Spectrum of Activity
Piperacillin/tazobactam has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections [see Indications and Usage (1) ].
Gram-positive bacteria:
Staphylococcus aureus (methicillin susceptible isolates only)
Gram-negative bacteria:
Acinetobacter baumannii
Escherichia coli
Haemophilus influenzae (excluding β-lactamase negative, ampicillin-resistant isolates)
Klebsiella pneumoniae
Pseudomonas aeruginosa (given in combination with an aminoglycoside to which the isolate is susceptible)
Anaerobic bacteria:
Bacteroides fragilis group (B. fragilis, B. ovatus, B. thetaiotaomicron, and B. vulgatus)
The following in vitro data are available, but their clinical significance is unknown.
At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for piperacillin/tazobactam. However, the safety and effectiveness of piperacillin/tazobactam in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria:
Enterococcus faecalis (ampicillin or penicillin-susceptible isolates only)
Staphylococcus epidermidis (methicillin susceptible isolates only)
Streptococcus agalactiae

Streptococcus pneumoniae

Streptococcus pyogenes

Viridans group streptococci
Gram-negative bacteria:
Citrobacter koseri
Moraxella catarrhalis
Morganella morganii
Neisseria gonorrhoeae
Proteus mirabilis
Proteus vulgaris
Serratia marcescens
Providencia stuartii
Providencia rettgeri
Salmonella enterica
Anaerobic bacteria:
Clostridium perfringens
Bacteroides distasonis
Prevotella melaninogenica
Susceptibility Testing Methods
As is recommended with all antimicrobials, the results of in vitro susceptibility tests, when available, should be provided to the physician as periodic reports, which describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution Techniques:
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of piperacillin and tazobactam powders.1,2 MIC values should be determined using serial dilutions of piperacillin combined with a fixed concentration of 4 μg/mL tazobactam. The MIC values obtained should be interpreted according to criteria provided in Table 7.
Diffusion Technique:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method1,3 and requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 100 mcg of piperacillin and 10 mcg of tazobactam to test the susceptibility of microorganisms to piperacillin/tazobactam. The disk diffusion interpreted criteria are provided in Table 7.
Anaerobic Techniques
For anaerobic bacteria, the susceptibility to piperacillin/tazobactam can be determined by the reference agar dilution method.4
| Susceptibility Test Result Interpretive Criteria | ||||||
|---|---|---|---|---|---|---|
| Minimal Inhibitory Concentration (MIC in mcg/mL) |
Disk Diffusion (Zone Diameter in mm) |
|||||
| Pathogen | S | I | R | S | I | R |
| Note: Susceptibility of staphylococci to piperacillin/tazobactam may be deduced from testing only penicillin and either cefoxitin or oxacillin. | ||||||
| Enterobacteriaceae | ≤ 16 | 32 – 64 | ≥ 128 | ≥ 21 | 18 – 20 | ≤ 17 |
| Acinetobacter baumannii | ≤ 16 | 32 – 64 | ≥ 128 | ≥ 21 | 18 – 20 | ≤ 17 |
|
Haemophilus influenzae
|
≤ 1 | - | ≥ 2 | ≥ 21 | - | - |
| Pseudomonas aeruginosa | ≤ 16 | 32 – 64 | ≥ 128 | ≥ 21 | 15–20 | ≤ 14 |
| Bacteroides fragilis group | ≤ 32 | 64 | ≥ 128 | - | - | - |
A report of S ("Susceptible") indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentration at the infection site necessary to inhibit growth of the pathogen. A report of I ("Intermediate") indicates that the results should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone, which prevents small, uncontrolled technical factors from causing major discrepancies in interpretation. A report of R ("Resistant") indicates that the pathogen is not likely to be inhibited even if the antimicrobial compound in the blood reaches the concentration usually achievable at the infection site; other therapy should be considered.
Quality Control
Standardized susceptibility test procedures require the use of quality controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test procedures.1,2,3,4 Standard piperacillin/tazobactam powder should provide the following ranges of values noted in Table 8. Quality control bacteria are specific strains of bacteria with intrinsic biological properties relating to resistance mechanisms and their genetic expression within the microorganism; the specific strains used for microbiological quality control are not clinically significant.
| Acceptable Quality Control Ranges | ||
|---|---|---|
| Minimum Inhibitory Concentration | Disk Diffusion | |
| QC Strain | Range (MIC in mcg/mL) | Zone Diameter Ranges in mm |
|
Escherichia coli
ATCC 25922 |
1 – 4 | 24 – 30 |
|
Escherichia coli
ATCC 35218 |
0.5 – 2 | 24 – 30 |
|
Pseudomonas aeruginosa ATCC 27853 |
1 – 8 | 25 – 33 |
|
Haemophilus influenzae
ATCC 49247 |
0.06 – 0.5 | 33 – 38 |
|
Staphylococcus aureus ATCC 29213 |
0.25 – 2 | - |
|
Staphylococcus aureus ATCC 25923 |
- | 27 – 36 |
Bacteroides fragilis
 ATCC 25285 |
0.12 – 0.5 | - |
Bacteroides thetaiotaomicron
 ATCC 29741 |
4 – 16 | - |
Clostridium difficile
 ATCC 700057 |
4 – 16 | - |
Eubacterium lentum
 ATCC 43055 |
4 – 16 | - |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals have not been conducted with piperacillin/tazobactam, piperacillin, or tazobactam.
Piperacillin/Tazobactam
Piperacillin/tazobactam was negative in microbial mutagenicity assays, the unscheduled DNA synthesis (UDS) test, a mammalian point mutation (Chinese hamster ovary cell HPRT) assay, and a mammalian cell (BALB/c-3T3) transformation assay. In vivo, piperacillin/tazobactam did not induce chromosomal aberrations in rats.
Piperacillin/Tazobactam
Reproduction studies have been performed in rats and have revealed no evidence of impaired fertility when piperacillin/tazobactam is administered intravenously up to a dose of 1280/320 mg/kg piperacillin/tazobactam, which is similar to the maximum recommended human daily dose based on body-surface area (mg/m2).
15 REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third Informational Supplement. CLSI document M100-S23, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2013.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Ninth Edition. CLSI document M07-A9, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Eleventh Edition. CLSI document M02-A11, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard - Eight Edition. CLSI document M11-A8. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, PA 19087 USA, 2012.
CLINITEST®is a registered trademark of Siemens Healthcare Diagnostics Inc.
16 HOW SUPPLIED/STORAGE AND HANDLING
ZOSYN® (piperacillin and tazobactam) for Injection are supplied as single-dose vials in the following sizes:
- Each ZOSYN 2.25 g vial provides piperacillin sodium equivalent to 2 grams of piperacillin and tazobactam sodium equivalent to 0.25 g of tazobactam. Each vial contains 5.58 mEq (128 mg) of sodium. Supplied 10 per box—NDC 0206-2501-10
- Each ZOSYN 3.375 g vial provides piperacillin sodium equivalent to 3 grams of piperacillin and tazobactam sodium equivalent to 0.375 g of tazobactam. Each vial contains 8.38 mEq (192 mg) of sodium. Supplied 10 per box—NDC 0206-3501-10
- Each ZOSYN 4.5 g vial provides piperacillin sodium equivalent to 4 grams of piperacillin and tazobactam sodium equivalent to 0.5 g of tazobactam. Each vial contains 11.17 mEq (256 mg) of sodium. Supplied 10 per box—NDC 0206-4501-10
ZOSYN® for Injection vials should be stored at controlled room temperature (20°C to 25°C [68°F to 77°F]) prior to reconstitution.
17 PATIENT COUNSELING INFORMATION
Patients should be counseled that antibacterial drugs including ZOSYN should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ZOSYN is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ZOSYN or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterial drugs which usually ends when the drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the drug. If this occurs, patients should contact their physician as soon as possible.

|
This product's label may have been updated. For current package insert and further product information, please visit www.-pfizer.com or call our medical communications department toll-free at 1-800-438-1985. |

|
Distributed by
Wyeth Pharmaceuticals Inc
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
 .
.
PREMIERPro™Rx is a trademark of Premier, Inc., used under license.
LAB-0712-1.0
PRINCIPAL DISPLAY PANEL - 3.375 gram Vial Label
NDC 0206-3501-01
3.375 gram Single Use Vial
Zosyn ®
(piperacillin and
tazobactam for
injection, USP)
3.375 gram
For IV Use Only
Rx only
PREMIERPro™ Rx

PRINCIPAL DISPLAY PANEL - 10 x 3.375 gram Vial Carton
NDC 0206-3501-10
Contains 10 vials of
NDC 0206-3501-01
Rx only
10 x 3.375 gram Single Use Vials
Zosyn®
(piperacillin and tazobactam for injection, USP)
3.375 gram
For IV Use Only
PREMIERPro™ Rx
PRINCIPAL DISPLAY PANEL - 2.25 gram Vial Label
NDC 0206-2501-01
2.25 gram Single Use Vial
Zosyn®
(piperacillin and
tazobactam for
injection, USP)
2.25 gram
For IV Use Only
Rx only
PREMIERPro™ Rx

PRINCIPAL DISPLAY PANEL - 10 x 2.25 gram Vial Carton
NDC 0206-2501-10
Contains 10 vials of
NDC 0206-2501-01
Rx only
10 x 2.25 gram Single Use Vials
Zosyn®
(piperacillin and tazobactam for injection, USP)
2.25 gram
For IV Use Only
PREMIERPro™ Rx
PRINCIPAL DISPLAY PANEL - 4.5 gram Vial Label
NDC 0206-4501-01
4.5 gram Single Use Vial
Zosyn ®
(piperacillin and
tazobactam for
injection, USP)
4.5 gram
For IV Use Only
Rx only
PREMIERPro™ Rx

PRINCIPAL DISPLAY PANEL - 10 x 4.5 gram Vial Carton
NDC 0206-4501-10
Contains 10 of
NDC 0206-4501-01
Rx only
10 x 4.5 gram Single Use Vials
Zosyn®
(piperacillin and tazobactam for Injection, USP)
4.5 gram
For IV Use Only
PREMIERPro™ Rx
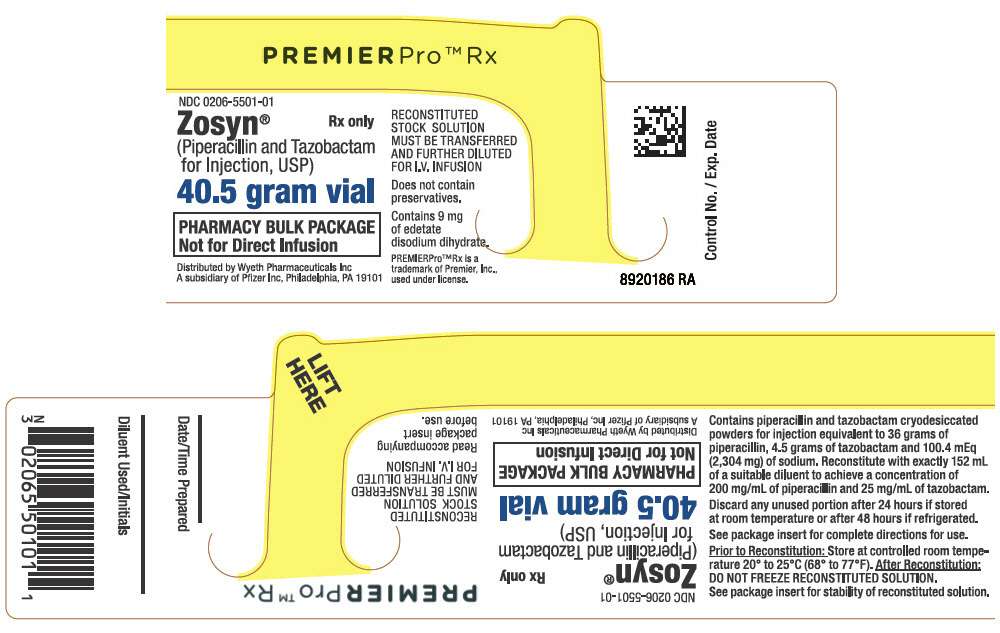
PRINCIPAL DISPLAY PANEL - 40.5 gram Vial Label
PREMIERPro™ Rx
NDC 0206-5501-01
Zosyn
®
(Piperacillin and Tazobactam
for Injection, USP)
Rx only
40.5 gram vial
PHARMACY BULK PACKAGE
Not for Direct Infusion
Distributed by Wyeth Pharmaceuticals Inc
A subsidiary of Pfizer Inc, Philadelphia, PA 19101
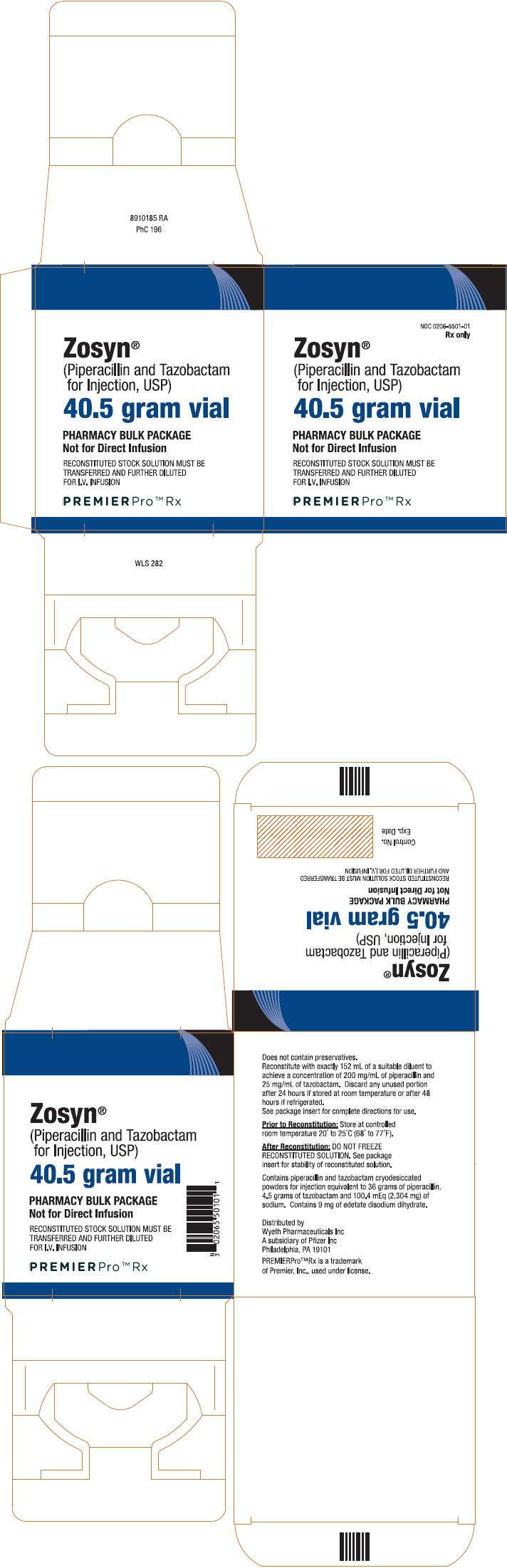
PRINCIPAL DISPLAY PANEL - 40.5 gram Vial Carton
NDC 0206-5501-01
Rx only
Zosyn
®
(Piperacillin and Tazobactam
for Injection, USP)
40.5 gram vial
PHARMACY BULK PACKAGE
Not for Direct Infusion
RECONSTITUTED STOCK SOLUTION MUST BE
TRANSFERRED AND FURTHER DILUTED
FOR I.V. INFUSION
PREMIERPro™ Rx
Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zosyntazobactam sodium and piperacillin sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||